Last year witnessed significant developments in False Claims Act enforcement, including a record-breaking number of new FCA cases. In this update, we cover recent developments in FCA jurisprudence, summarize significant enforcement activity, and analyze the most notable legislative, policy, and caselaw developments from the second half of calendar year 2024, picking up where our mid-year 2024 update left off.
Last year, the Department of Justice (DOJ) and qui tam relators smashed 2023’s all-time record for the number of newly filed False Claims Act (FCA) cases. While monetary recoveries from FCA cases in 2024 remained largely in line with trends over the last decade, 2024’s figure was still the highest in three years, coming in just shy of $3 billion. As in previous years, the lion’s share of this figure (approximately $2.2 billion) came from qui tam suits filed by private relators where DOJ subsequently intervened.
Another noteworthy 2024 development threatens to derail this enforcement train. In September 2024, a Florida federal court ruled the FCA’s qui tam provisions unconstitutional under the Appointments Clause. Ordinarily, that decision, in United States ex rel. Zafirov v. Florida Medical Associates, LLC, would seem unlikely to survive the Eleventh Circuit’s scrutiny, given the other cases previously holding that qui tam suits are constitutional. But Zafirov tracks statements by three Supreme Court Justices last year that they had questions about the constitutionality of the qui tam provision. Regardless of the outcome of that case, the robust pace of current enforcement efforts—both DOJ- and relator-led—is likely to continue for the foreseeable future. In this update, we cover both the Zafirov decision and other recent developments in FCA jurisprudence, and consider their implications for companies facing FCA matters that have progressed to litigation.
Last year’s record statistics also serve as a reminder that, while DOJ’s FCA enforcement priorities can shift after a presidential administration transition, it takes far more than a change in the political climate to slow FCA enforcement. In this update, we share our insights on how the second Trump Administration DOJ may distinguish its FCA enforcement efforts from those of the Biden Administration (and the first Trump Administration). And we also assess how relator-led cases are likely to continue to expand potential enforcement theories, forcing DOJ to crystallize its enforcement priorities via its intervention decisions.
This update also summarizes significant enforcement activity and analyzes the most notable legislative, policy, and caselaw developments from the second half of calendar year 2024, picking up where our last update left off. You can find all of Gibson Dunn’s publications regarding the FCA on our website, including a detailed discussion of how the FCA operates, industry-specific presentations, and practical guidance for companies seeking to navigate FCA enforcement.
I. FCA ENFORCEMENT ACTIVITY
A. NEW FCA ACTIVITY
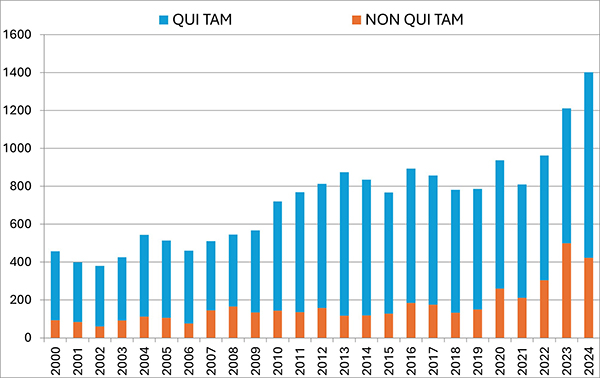
In 2024, FCA enforcement reached staggering new heights—higher, even, than the then-record-setting number of new FCA cases in 2023. The government and qui tam relators filed 1,402 new cases in 2024, representing an additional 190 additional cases (a jump of about 16%) beyond 2023’s previous record total of 1,212 total new cases.
Of these new cases, relators initiated a record 979 qui tam actions in FY 2024—a 37% increase over the prior year, and a number far in excess of the prior record of 757 actions brought in 2013. The 979 new qui tam cases exceed the total number of FCA actions (both qui tam and non-qui tam) brought in all years except 2023.
The government, meanwhile, initiated 423 FCA matters in FY 2024. This is the second-highest number since 1986 (surpassed only by last year’s total of 505) and reflects DOJ’s continued investment in identifying leads for FCA enforcement without the assistance of relators.
For companies trying to anticipate developments in FCA enforcement under the second Trump Administration, FY 2024’s record number of new FCA actions highlights an important reality: regardless of the extent to which overall enforcement priorities evolve in the next four years, the sheer number of pending FCA cases will inevitably shape enforcement dynamics in the near term while these cases wend their way through the investigative and judicial processes. Further, DOJ’s intervention decisions in qui tam cases filed during the prior administration will go a long way toward revealing enforcement priorities going forward.
Number of FCA New Matters, Including Qui Tam Actions
 |
Source: DOJ “Fraud Statistics – Overview” (Jan. 15, 2025).
B. TOTAL RECOVERY AMOUNTS
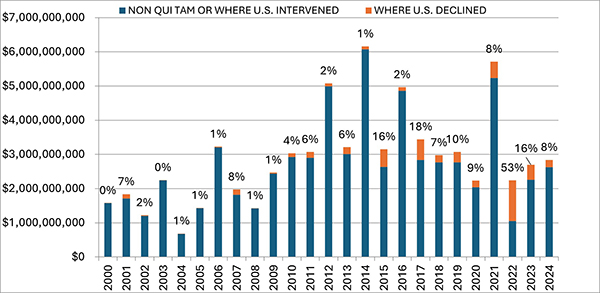
In FY 2024, settlements and judgments under the FCA resulted in over $2.9 billion in recoveries, a figure just slightly above last year’s recovery of $2.7 billion. FY 2024’s total is broadly in line with yearly totals dating back to 2017, suggesting that 2021’s all-time record of $5.7 billion may be an outlier.
Notably, the government’s already sizeable recoveries in FY 2024 do not include two large settlements DOJ announced after the close of its 2024 fiscal year. In mid-October 2024, DOJ announced a $425 million settlement with a pharmaceutical company and a $428 million settlement with a defense contractor; the latter, according to DOJ, is the second largest government procurement-related recovery in FCA history. Together, these two settlements already bring FY 2025’s running total to over $850 million, suggesting that FY 2025 could surpass FY 2024 and mark the fourth straight year of increasing recoveries.
Settlements or Judgments in Cases Where the Government Declined Intervention as a Percentage of Total FCA Recoveries
 |
Source: DOJ “Fraud Statistics – Overview” (Jan. 15, 2025).
C. FCA RECOVERIES BY INDUSTRY
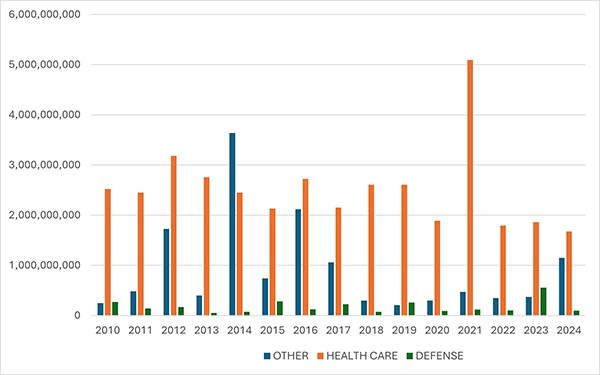
In keeping with prior years, most recoveries under the FCA in 2024 came from the health care sector, which saw approximately $1.7 billion in settlements and judgments. That figure represents a slight decrease from last year—and is in fact the lowest amount since 2009.
On the other hand, settlements and judgments in DOJ’s “Other” category (i.e., non-health care, non-defense) tripled from $370 million in FY 2023 to approximately $1.15 billion in FY 2024. Major recoveries in this area included a $38.2 million settlement agreement with a city government regarding its receipt of federal Department of Housing and Urban Development grant funds.
FCA Recoveries by Industry
 |
Source: DOJ “Fraud Statistics – Health and Human Services”; “Fraud Statistics – Department of Defense”; “Fraud Statistics – Other (Non-HHS and Non-DoD)” (Jan. 15, 2025).
II. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE SECOND HALF OF 2024
A. HEALTH CARE AND LIFE SCIENCES INDUSTRIES
- On July 1, two home health agencies and their parent entity agreed to pay approximately $4.5 million to resolve allegations that they provided illegal kickbacks to providers in exchange for Medicare referrals, in violation of the Anti-Kickback Statute (AKS) and the FCA. The government claimed that the agencies provided kickbacks in the form of lease payments, wellness health services, sports tickets, and meals. DOJ awarded the agencies an unspecified amount of credit for self-disclosure and cooperation with the government’s investigation, including in the form of assistance in determining the government’s alleged losses.[1]
- On July 10, a pharmacy chain and three subsidiaries agreed to settle claims that they violated the FCA by improperly reporting rebates received by drug manufacturers as service fees to the Centers for Medicare and Medicaid Services (CMS). As part of the settlement, the pharmacy and one of its subsidiaries will pay $101 million to the federal government. Further, the pharmacy’s other two subsidiaries will grant the United States an allowed, unsubordinated, general unsecured claim of $20 million in the pharmacy’s bankruptcy case in the District of New Jersey. The settlements resolved a qui tam suit brought by a former employee.[2]
- On July 10, a health care provider and twelve affiliated skilled nursing facilities agreed to pay $21.3 million to resolve allegations that they submitted false claims for rehabilitation therapy services. The government alleged that the provider created a quota system that incentivized employees to bill Medicare for therapy services that were “unreasonable, unnecessary, unskilled, or that simply did not occur as billed.” The government further alleged that the provider submitted false claims to Medicaid by using an inflated reimbursement rate that relied on data inaccurately reflecting the type and degree of care needed by the patients. The settlement resolved a qui tam lawsuit brought by two former employees of the company. In connection with the settlement, the provider entered into a five-year corporate integrity agreement (CIA) with the Department of Health and Human Services Office of Inspector General (HHS-OIG) that requires an independent review organization to assess annually the medical necessity and appropriateness of therapy services billed to Medicare.[3]
- On July 11, a pharmacy chain and 10 subsidiaries agreed to settle allegations that they violated the FCA by knowingly dispensing unlawful prescriptions for opioids. According to the government, the prescriptions lacked a legitimate medical purpose, were not issued in the usual course of professional practice, were not for a medically accepted indication, or were medically unnecessary or otherwise invalid. As part of the settlement, the government will be paid $7.5 million and have an allowed, unsubordinated, general unsecured claim of $401.8 million in the pharmacy’s bankruptcy case in the District of New Jersey. The settlement resolves claims brought in a qui tam suit by former pharmacy employees. In conjunction with the settlement, the pharmacy chain entered into a Memorandum of Agreement (MOA) with the Drug Enforcement Administration (DEA), which includes employee training requirements and imposes certain recording-keeping obligations on the pharmacy for five years. The pharmacy chain also entered into a CIA with HHS-OIG that requires an independent review organization to determine whether prescription drugs are properly prescribed, dispensed, and billed.[4]
- On July 12, a provider of biopharmaceutical research services agreed to pay $5.38 million to settle allegations that it violated the FCA and the AKS. The government alleged that the company paid commissions to independent marketers in exchange for recommending the company’s services, and then submitted reimbursement claims for those services to Medicare and Medicaid. The government also alleged that the company continued to pay commissions to independent marketers even after learning that such payments violated the AKS. The settlement resolved a qui tam suit brought by two co-founders of a former independent marketer for the company. The company separately agreed to pay $147,851 to individual states for reimbursement claims paid by state Medicaid programs.[5]
- On July 18, a hospice center and its affiliates agreed to pay more than $19.4 million to resolve FCA claims premised on the alleged knowing submission of false claims for hospice services for patients who were not terminally ill or were otherwise ineligible for hospice care. The settlement also resolved nine qui tam lawsuits brought by current and former employees.[6]
- On July 18, a kidney dialysis provider agreed to pay over $34.4 million to resolve allegations that it improperly induced referrals to a former subsidiary that provided pharmacy services to dialysis patients. The government also alleged that the provider paid purported kickbacks to physicians to induce referrals to the company’s dialysis centers. This settlement resolved a qui tam suit brought by a former Chief Operating Officer of the provider.[7]
- On August 1, a Florida county agreed to pay $3.5 million to settle FCA allegations of fraudulent billing by the county’s Emergency Medical Service department. Specifically, the government alleged that over a seven-year period, the county billed federal health care programs for emergency medicine and transportation services performed by technicians who lacked appropriate certifications. The settlement also resolves a qui tam suit brought by the medical director of the county’s Emergency Medical Service.[8]
- On August 12, a Texas medical group and its founder and CEO agreed to pay a total of $8.9 million to settle allegations that they submitted false claims to Medicare by offering and providing illegal remuneration to physicians to induce referrals to their surgical centers, in violation of the AKS. The settlement resolves claims brought in a qui tam suit by a radiologist.[9]
- On August 20, a home health care and hospice provider and its subsidiaries agreed to pay approximately $3.8 million to resolve claims that the provider knowingly submitted false Medicare claims for patients who were ineligible for home health care or hospice benefits, for services that were unreasonable or not medically necessary, for services performed by untrained staff, or for services that were never performed. The settlement also resolved two additional qui tam suits brought by a former travel nurse, former managers, and former directors.[10]
- On August 26, a Missouri-based company specializing in durable medical equipment agreed to pay $13.5 million to resolve allegations that the company violated the FCA when it knowingly submitted claims based on patient evaluations that were unlawfully authored, completed, or signed by company employees rather than qualified medical professionals. The settlement agreement resolves three qui tam suits brought by employees or former employees. In agreeing to the settlement amount, the government considered the company’s self-disclosure of several overpayments and its cooperation with the government’s investigation.[11]
- On August 27, a Montana health care system agreed to pay $10.8 million to resolve allegations that it submitted false claims related to services performed by an oncologist. Over a five-year period, the health care system allegedly submitted false claims for the oncologist’s services that it knew or should have known were coded at a higher level than what was performed or did not meet requirements to constitute separate identifiable services. Additionally, the government alleged that the oncologist’s salary was inconsistent with fair market value because it was calculated in reliance on the alleged false documentation and certifications regarding the oncologist’s services. The health care system received an unspecified amount of credit for undertaking an internal investigation, voluntarily self-disclosing the doctor’s misconduct, significantly cooperating with the government (including by providing documents it was not legally required to produce and making relevant individuals available for interviews), and enhancing its corporate compliance program. The settlement agreement specifies that $9,988,970.15 of the settlement is restitution, amounting to just under a 1.1x multiplier if the restitution amount specified in the resolution is treated as a proxy for single damages.[12]
- On September 4, a generic pharmaceutical manufacturer agreed to pay $25 million to settle allegations under the FCA that it conspired to fix the price of a generic drug. According to the government, the company violated the AKS by making and receiving payments in connection with arrangements with other pharmaceutical manufacturers on price, supply, and customer allocation. The company previously entered into a $30 million deferred prosecution agreement with DOJ to resolve criminal antitrust charges stemming from the same conduct. DOJ’s press release makes clear that the FCA settlement amount “is in addition to the criminal penalty paid by the company,” meaning the government did not offset one amount against the other.[13]
- On September 13, a major U.S. pharmacy retailer and its parent holding company agreed to pay $106.8 million to resolve allegations that the pharmacy violated the FCA and related state statutes by billing government health care programs for prescriptions that were never dispensed to patients. The government alleged that over an eleven-year period, the pharmacy restocked and resold prescriptions to beneficiaries that had initially been filled for other beneficiaries who failed to pick up the prescriptions. The pharmacy obtained a very favorable settlement, including credit for self-disclosure of certain conduct, cooperation, and remediation of its electronic pharmacy management system, as well as credit for $66 million that it had already refunded to the government.[14]
- On September 16, a surgery center and its affiliates agreed to pay approximately $12.76 million to resolve alleged FCA violations stemming from improper financial relationships between the surgery center and two physician groups. The government alleged that the surgery center made improper financial contributions to the physician groups in violation of the FCA, AKS, and Stark Law, by funding athletic trainers’ salaries in exchange for referrals by the trainers to the center. As part of the settlement, approximately $12.76 million will be paid to the federal government, and South Dakota, Iowa, and Nebraska will collectively receive approximately $1.37 million. The government acknowledged that the surgery center took significant steps entitling it to credit for cooperating with the government.[15]
- On September 18, a provider agreed to pay $60 million to resolve allegations that it made payments to third-party insurance agents in return for referrals of Medicare Advantage beneficiaries, in violation of the AKS and the FCA. The government claimed that the provider’s conduct caused the submission of false claims to Medicare Advantage organizations and in turn to the federal government. The settlement resolves a qui tam lawsuit.[16]
- On September 26, a health care company that owns and operates inpatient behavioral-health facilities in multiple states agreed to pay $19.85 million to resolve allegations that it submitted false claims to government health care programs. According to the government, the company submitted claims for unreasonable and medically unnecessary behavioral health services, including improperly admitting and failing to discharge patients who either never required or no longer needed inpatient care. Further, the government alleged the company knowingly failed in its training, supervision, and scheduling of staff, resulting in significant harm to patients, and failed to meet regulatory obligations for developing plans for assessments, treatment, and discharge and to provide required patient therapy. The federal government will receive around $16.66 million of the recovery, while Florida, Georgia, Michigan, and Nevada will collectively receive $3.18 million. The settlement resolves qui tam suits brought by former employees of the health care company.[17]
- On September 30, two Brooklyn-based licensed home-care service agencies agreed to pay $3.9 million to the United States and $5.85 million to New York State to resolve allegations that the companies used Medicaid payments to pay the wages and benefits of its home health aides, violating the federal FCA and New York State’s FCA by claiming that they paid the minimum wages required under New York state law.[18]
- On September 30, a behavioral health organization in Massachusetts agreed to pay at least $5.5 million and up to $6.5 million to resolve allegations that it violated the federal FCA and the Massachusetts False Claims Act. The settlement resolved allegations that the company provided free sober housing to substance use recovery patients enrolled in Medicare and Medicaid to induce these patients to participate in its program, in violation of the federal and Massachusetts Anti-Kickback Statutes. The allegations underlying the settlement agreement stemmed from a qui tam suit brought by the president of a company with whom the behavioral health organization contracted for patient housing.[19]
- On October 2, a toxicology laboratory agreed to pay $27 million to resolve claims that it billed federal health care programs for medically unnecessary urine tests and provided physicians with free items in exchange for testing referrals. The United States will receive $18.2 million of the settlement amount. The remainder will be paid to affected states, including Maryland, Illinois, Minnesota, Virginia, Georgia, and Colorado. This settlement resolves a qui tam suit.[20] As part of the settlement, the laboratory entered a five-year CIA with HHS-OIG that mandates engagement of an independent review organization to ensure that claims submitted to Medicaid or Medicare for reimbursement are properly coded, submitted, and reimbursed, and that the items and services involved are medically necessary. The CIA also mandates the appointment of a compliance officer with prescribed oversight responsibilities, and the creation of an anonymous whistleblower program for reporting potential violations.[21]
- On October 3, a Medicaid call center operator agreed to pay $11.36 million to resolve allegations that it violated the FCA by fraudulently reporting call center performance metrics. The Company faced up to $26 million in FCA liability due to the conduct of two employees who, from 2018 to 2023, allegedly submitted fabricated call center performance metrics and adjusted invoices to the South Carolina Department of Health and Human Services. In entering into the settlement, the government took into consideration the call center operator’s voluntary disclosure and remedial action.[22]
- On October 4, a medical center agreed to pay $14.2 million to settle FCA allegations that it violated Medicare regulations and the Stark Law, which prohibits physician self-referrals. The government alleged that the medical center submitted claims for Medicare reimbursement that did not include a “PN” modifier indicating that the services were provided at a non-excepted off-campus outpatient facility. Further, according to the government, the medical center maintained agreements that created financial relationships between the hospital and physician-owners who referred Medicare beneficiaries to the hospital in violation of the Stark Law. The medical center self-reported the alleged conduct to DOJ. As part of its disclosure, the medical center provided an independent third-party expert’s analysis of the financial impact of the omitted “PN” modifiers and disclosed the existence of its various agreements related to physical referrals. The settlement agreement credits the medical center for its voluntary self-disclosure and subsequent cooperation with investigators. Of the total settlement amount, $9.46 million (approximately 66%) constituted restitution.[23]
- On October 10, a generic pharmaceutical manufacturer agreed to pay $25 million to settle allegations under the FCA that the company conspired to fix prices and allocate markets for two generic drugs. This settlement constituted one part of a larger resolution with the company totaling $450 million. The government specifically alleged that the company violated the AKS by making and receiving payments in connection with arrangements with other pharmaceutical manufacturers on price, supply, and customer allocation. The company previously entered into a deferred prosecution agreement with DOJ to resolve criminal antitrust charges stemming from the same conduct. This was the second FCA resolution in as many months in which the Eastern District of Pennsylvania U.S. Attorney’s Office settled on an antitrust-related theory. As part of the broader $450 million settlement, the company agreed to pay $425 million to resolve allegations that it paid kickbacks via two co-pay assistance foundations in violation of the AKS and the FCA.[24]
- On November 1, a supplier of compound prescription ingredients agreed to pay $21.75 million to resolve allegations that it violated the FCA by falsely inflating its Average Wholesale Prices (AWPs) for certain ingredients used to fill prescriptions reimbursed by the TRICARE program. The government alleged “spreads” of hundreds (and in one case, thousands) of dollars as between reported AWP and the price at which the supplier bought and sold the ingredients. The government claimed that the supplier used these spreads to induce pharmacy customers to purchase the ingredients from the supplier given the profit potential that the spreads created for the pharmacies. This settlement resolves the qui tam suit underlying the settlement.[25]
- On November 1, a pharmaceutical manufacturer and its CEO agreed to pay $47 million to resolve allegations that they caused the submission of false claims to federal health care programs, in violation of the FCA, by offering kickbacks. The government alleged that the manufacturer distributed breath test kits to health care providers to give to patients and then paid a laboratory to analyze and report the results to the manufacturer, which results the manufacturer then allegedly disseminated to its sales force. The federal portion of the recovery amounts to approximately $43.6 million, while the remainder constitutes a recovery for state Medicaid programs. The allegations resolved by the settlement agreement were, in part, originally raised in a qui tam suit brought by former employees of the manufacturer.[26] In connection with the settlement, the manufacturer and its CEO entered into a CIA with HHS-OIG, which requires the establishment of a compliance program, the engagement of an independent review organization, and the development and implementation of a centralized annual risk assessment and internal review process.[27]
- On November 7, a Kentucky-based laboratory agreed to pay $6.5 million to resolve allegations that it submitted false claims for payment to Medicare for urine drug testing and for its proprietary test for chronic pain. The government alleged that the laboratory submitted multiple claims for the testing for the same patient, on the same date of service, using the same urine sample. The settlement agreement further states that the laboratory submitted claims for testing for chronic pain for patients without any individualized determination of medical necessity by the ordering provider.[28] In connection with the settlement, the company also entered into a five-year CIA with HHS-OIG. The CIA requires that the company engage an independent review organization to ensure that claims submitted to Medicaid for reimbursement are properly coded, submitted, and reimbursed, and that the items and services involved are medically necessary. The CIA also mandates the appointment of a compliance officer with prescribed oversight responsibilities, and the creation of an anonymous whistleblower program for reporting potential violations.[29]
- On November 12, a hospital network agreed to pay $23 million to resolve allegations that it falsely coded certain evaluation and management claims submitted to federal Medicare and TRICARE programs. Specifically, the government alleged that the hospital network automatically used a certain code every time a provider checked a patient’s set of vital signs more times than the total number of hours the patient was in the emergency room. The government alleged that the automatic use of this code did not appropriately reflect the utilization of hospital resources and therefore violated Medicare billing requirements. The settlement resolved a qui tam suit.[30]
- On November 21, a California hospital agreed to pay $10.25 million to resolve FCA allegations of a kickback and self-referral scheme to increase hospital admissions. The hospital allegedly paid a bonus to physicians based on the number of patients they admitted, and then submitted knowingly false claims to Medicare and Medi-Cal (California’s Medicaid program) for medically unnecessary hospital admissions. The settlement agreement also resolved allegations that the hospital knowingly admitted patients when it knew inpatient care was not medically necessary and submitted claims that included false diagnosis codes. Under the settlement, the hospital paid approximately $9.5 million to the federal government, of which nearly $4.8 million was restitution; and approximately $730,000 to the State of California. The settlement resolved a qui tam suit.[31] In connection with the settlement, the hospital entered into a five-year CIA with HHS-OIG that requires the implementation of a risk assessment and internal review process and an annual review by an independent review organization to assess the necessity and appropriateness of Medicare claim submissions and systems to track arrangements with referral sources.[32]
- On December 11, a management consulting firm agreed to pay over $323 million to settle allegations under the FCA for allegedly providing advice to a pharmaceutical company that caused the submission of false and fraudulent claims to federal health care programs for medically unnecessary prescriptions of opioids, as well as allegedly failing to disclose to the U.S. Food and Drug Administration (FDA) conflicts of interest arising from the firm’s concurrent work for the pharmaceutical company and the FDA. Alongside the civil settlement, the management consulting firm entered into a five-year CIA with HHS-OIG focused on risk assessment and quality control. This is the first CIA that the HHS-OIG has entered into with a management consulting firm. The firm also entered into a parallel criminal resolution.[33]
- On December 20, a health insurance provider and its affiliate agreed to pay $98 million to settle an FCA case related to Medicare Part C. Under Medicare Part C, CMS reimburses health insurance providers for certain plans based partly on the “risk score” of the beneficiaries, which in turn is based on demographic and diagnosis information. The government alleged that the provider in this case had created a wholly owned subsidiary to retrospectively search medical records and query physicians for information to support additional diagnoses that would increase beneficiaries’ risk scores for purposes of Medicare Part C reimbursement. The beneficiaries’ medical records allegedly did not support the additional diagnoses or higher risk scores. Under the settlement agreement, the provider will make guaranteed payments of $34.5 million and contingent payments of up to $63.5 million based on its ability to pay. In connection with the settlement, the provider entered into a five-year Corporate Integrity Agreement with HHS-OIG. The CIA requires that the provider hire an independent review organization to review annually a sample of the provider’s patients’ medical records as well as associated internal controls to ensure appropriate risk adjustment payments. The settlement resolves a qui tam suit brought by a former employee of another insurance provider.[34]
- On December 20, sixteen cardiology practices agreed to pay a total of nearly $17.8 million to resolve claims that they overbilled Medicare for diagnostic radiopharmaceuticals used for cancer detection and treatment. The government alleged that over a period of at least a year and for some providers, more than ten years, the cardiology practices overinflated the acquisition costs of radiopharmaceuticals based on their actual costs, in contravention of Medicare Part B guidance. The settlements ranged in value from $50,000 for one of the practices to $6.75 million for another of the practices. These settlements resolve qui tam The qui tam suits stemmed from data mining practices that originally identified hundreds of defendants.[35] One of the cardiology practices entered into a CIA with HHS-OIG, which requires the engagement of an independent review agency to review the practice’s fee-for service Medicare claims to confirm medical necessity, appropriate documentation, and proper coding.[36]
- On December 20, a pharmacy agreed to pay approximately $8.2 million to resolve allegations that it violated the FCA by billing for COVID-19 tests that were not actually provided to Medicare beneficiaries. The investigation arose out of a demonstration project CMS conducted whereby Medicare Part B providers could secure reimbursement for up to eight over-the-counter COVID-19 tests per Medicare Part B beneficiary. The government claimed that the pharmacy billed for tests without shipping the tests to beneficiaries.[37]
- On December 23, a regional health insurance provider agreed to pay $15.23 million to resolve allegations that it provided gift cards to administrative service providers to induce the referral and enrollment of Medicare beneficiaries into the insurer’s Medicare Advantage plan, in violation of the AKS and the FCA. The settlement agreement was accompanied by a CIA with HHS-OIG, which required that the insurer to institute a compliance program and put in place processes to avoid marketing arrangements that violate AKS and engage an independent organization to annually review these procedures.[38]
- On December 23, a regional grocery store chain headquartered in Virginia agreed to pay approximately $8 million to resolve allegations that store pharmacies dispensed controlled substances, including opioids, that were medically unnecessary, lacked a legitimate medical purpose or medically accepted indication, and/or were not dispensed pursuant to valid prescriptions. The civil settlement includes the resolution of claims brought under the qui tam of the FCA.[39]
- On December 26, a California-based medical center and laboratory, along with the physician-owner and an executive, agreed to pay $15 million to resolve allegations that they submitted false claims to Medicare and Medi-Cal by paying kickbacks to Medicare and Medi-Cal beneficiaries and third-party clinics for patient referrals and referring those same patients to the lab, in violation of the Stark Law, AKS, and FCA. The allegations underlying the settlement agreement stemmed from a qui tam suit brought by four former employees and managers of the medical center and lab.[40]
B. GOVERNMENT CONTRACTING AND PROCUREMENT
- On July 31, a biotech company agreed to pay $5 million to settle allegations under the FCA that its subsidiary fraudulently overcharged federal agencies for scientific and technical laboratory supplies. The government specifically alleged that the subsidiary violated “Most Favored Customer Pricing” provisions and other pricing terms in contracts with the Department of Defense, Department of Veterans Affairs (VA), and National Institutes of Health. The company acquired the subsidiary in 2017; however, the subsidiary’s conduct covered by the agreement allegedly occurred between 2008 and 2017. The settlement resolved a qui tam suit brought by a former company employee.[41]
- On October 16, a defense contractor agreed to pay $428 million to settle allegations that the company violated the FCA by knowingly failing to provide truthful certified cost and pricing data during negotiations on numerous government contracts, in violation of the Truth in Negotiations Act. The company received credit in the settlement for its cooperation and remediation efforts. The company also entered into a parallel $147 million criminal resolution with DOJ.[42]
- On November 5, a federal government contractor for protective security guard services agreed to pay $52 million to settle allegations that the company violated the FCA by knowingly causing entities that it controlled to fraudulently obtain U.S. Department of Homeland Security set-aside contracts reserved for small businesses. The settlement further resolved allegations that the arrangements with the purported small businesses violated the Anti-Kickback Act, which prohibits kickbacks in exchange for favorable treatment in connection with government procurement efforts. The allegations underlying the settlement agreement stemmed from a qui tam suit brought by the CEO and President of another government contractor.[43]
- On November 19, two technology companies agreed to pay $2.3 million and $2.05 million, respectively, to resolve FCA allegations premised on a scheme to submit non-competitive contract bids. DOJ alleged that one company gave the other company advantageous pricing to sell products to the Army, and then the first company submitted its own direct bids to create the illusion of competition. The allegations against one of the companies were the subject of a qui tam suit.[44]
- On December 19, an international development contractor agreed to pay approximately $3.1 million to resolve allegations that it submitted fraudulent claims for payment to the U.S. Agency for International Development (USAID). Specifically, the government claimed that the contractor billed USAID for charges fraudulently submitted to the contractor by its own subcontractor, without the contractor detecting the issue. DOJ credited the company for taking “a number of significant steps” in the course of the investigation.[45]
- On December 31, an information technology and professional services contractor headquartered in Newport News, Virginia, agreed to pay $2.63 million to resolve claims under the FCA that it misrepresented to the General Services Administration that it was eligible for certain small business set-aside contracts. The government alleged that the company attempted to qualify for the set-asides (which had eligibility criteria based on average revenue) by novating a contract to another company and then misrepresenting that the two companies were unrelated. The government alleged that the companies were in fact linked because, among other reasons, the owners of each company were married, and the two companies shared executives.[46]
C. CUSTOMS, FINANCIAL INDUSTRY, AND MISCELLANEOUS FEDERAL FUNDING
- On August 7, a Texas-based company agreed to pay $2.05 million—of which $1.02 million is restitution—to resolve allegations that the company improperly obtained Post 9/11 GI Bill funding through its operation of vocational schools. The government alleged that the company enrolled VA-supported veterans in courses where more than 85% of the students had at least some of their costs paid by the school or the VA; this allegedly violated the 85/15 rule, which was meant to ensure that veterans were enrolled in quality courses by weeding out those programs which depend on federal funds to remain afloat.[47]
- On August 8, two Wisconsin-based companies and their two principals agreed to pay a total of $10.2 million to settle allegations that they failed to pay millions in customs duties on goods imported from China. The government alleged that for five years, the companies engaged in an undervaluation scheme by providing falsified invoices to their customs broker (with the actual prices of imported goods typically reduced by 70%), who then unknowingly submitted these false invoices to customs officials. The settlement resolves a related qui tam suit brought by a former employee. DOJ’s announcement of the resolution explains that $4.2 million of the total settlement amount was paid to U.S. Customs and Border Protection before the DOJ settlement; this may help explain why the relator’s share was quite low compared to the full $10.2 million.[48]
- On August 9, a women’s apparel company agreed to pay $7.6 million to resolve allegations that it underpaid customs duties over a seven-year period by falsely representing to customs officials the true value of its apparel imports. Specifically, the government alleged that the company failed to include the value of certain fabric and garment trims in the value of the imports, failed to report discrepancies it discovered on customs forms, and made additional errors in classifying textiles and providing port of entry codes. The government noted the apparel company’s voluntary and timely disclosure of relevant evidence and the company’s efforts to prevent future issues through training and compliance measures. The settlement resolves a qui tam suit.[49]
- On August 26, a California city agreed to pay $38.2 million to resolve allegations that it knowingly misrepresented its compliance with certain federal housing development grant requirements by failing to follow federal accessibility laws when building and rehabilitating affordable multifamily properties and failing to make its affordable multifamily housing program accessible to people with disabilities. The allegations underlying the settlement agreement stemmed from a qui tam suit brought by a city resident.[50]
- On September 18, a Missouri-based former mortgage lender agreed to pay $2.4 million to resolve allegations that it violated the FCA and the Financial Institutions Reform, Recovery, and Enforcement Act by knowingly underwriting Home Equity Conversion Mortgages (HECM) insured by the Department of Housing and Urban Development’s Federal Housing Administration (FHA) that did not meet program eligibility requirements. The FHA offers the HECM as a reverse mortgage program specifically for senior homeowners aged 62 and older. The government alleged that the mortgage lender knowingly violated underwriting requirements by allowing inexperienced temporary staff to underwrite FHA-insured loans; the government also asserted that the lender submitted loans for FHA insurance with underwriter signatures that were falsified or affixed before all the documentation the underwriter should have reviewed was complete.[51]
D. PAYCHECK PROTECTION PROGRAM (PPP) RESOLUTIONS
After entering into a large PPP-related settlement in May 2024 (as covered in our Mid-Year 2024 FCA Update), DOJ secured a number of smaller settlements resolving FCA allegations related to PPP eligibility criteria in the second half of 2024.
- On July 17, a GPS manufacturer agreed to pay $2.6 million to settle FCA allegations that it made false certifications regarding its ties to the People’s Republic of China, rendering it in fact ineligible for the PPP loan it received and later had forgiven.[52]
- On August 8, a New Jersey non-profit health system agreed to pay $3.15 million to settle FCA claims that it was affiliated with a larger health care system and thus was ineligible for a PPP loan.[53]
- On August 8, a California-based company operating a network of dental offices in Southern California, along with its founders and former owners, agreed to pay $6.3 million to resolve FCA allegations that they misrepresented their affiliations—and, thus, the network’s size—when applying for PPP loans.[54]
- On September 17, a Florida-based consulting company specializing in travel and tourism agreed to pay approximately $2.28 million to settle FCA claims that it obtained a PPP loan without filing a required registration statement under the Foreign Agents Registration Act (FARA).[55]
- On October 22, a California agricultural association and its chief executive officer agreed to pay approximately $5.66 million to resolve FCA allegations that the association was not eligible for the PPP loan that it obtained because the association was government owned.[56]
- On December 13, a Wisconsin-based subsidiary of a Swiss machinery cutting and software company agreed to pay $2.3 million to resolve FCA allegations that the company’s affiliation with its Swiss parent made it too large—in terms of employee headcount—to obtain a PPP loan.[57]
III. LEGISLATIVE AND POLICY DEVELOPMENTS
A. FEDERAL POLICY AND LEGISLATIVE DEVELOPMENTS
1. Issues to Watch During the New Administration
Combating fraud on the government fisc has historically had bipartisan appeal. Typically, when the White House changes hands between political parties, the pace of FCA enforcement may change at the margins but rarely changes by an order of magnitude. What often does change is the relative balance of enforcement priorities within the FCA sphere—the policy agenda that is set by appointed officials and that forms the framework in which career DOJ attorneys perform the day-to-day work of investigating and litigating cases. More often than not, companies trying to discern a new administration’s enforcement agenda in early days are left to rely on the pronouncements of newly appointed officials.
Given President Trump’s status as the only modern U.S. president to serve non-consecutive terms, however, FCA developments during his first term provide a natural starting place for highlighting areas to watch over the next four years. Chief among these are cybersecurity, sub-regulatory guidance, and voluntary dismissal of qui tam cases.
In October 2021, DOJ announced the Civil Cyber-Fraud Initiative, which has since been a key focus of FCA actions pursued for the last four years.[58] The Initiative was emblematic of the Biden Administration’s emphasis on cybersecurity issues as part of its broader National Cybersecurity Strategy. Under the Initiative, DOJ pursued FCA recoveries from companies for alleged fraudulent noncompliance with cybersecurity requirements, entering into a number of settlements and filing its first lawsuit against a federal contractor under the Initiative this past August. At this juncture, it is not entirely clear whether the Trump Administration will continue with the Initiative or otherwise focus on cybersecurity cases. However, cybersecurity tends to be a bi-partisan issue, with major developments in this area occurring in each of the last three administrations, including President Trump’s first term. Relators also are likely to continue filing qui tam complaints in this area, automatically triggering investigations of their allegations. Moreover, given recent high-profile cybersecurity attacks against U.S. interests—including President Trump’s presidential campaign[59] and at least one major federal contractor[60]—there is a good chance that cybersecurity enforcement will continue to be an area of focus.
On the other hand, premising FCA liability on sub-regulatory guidance may lose favor under the new Trump Administration, if the past is any guide. It was under the first Trump Administration that DOJ issued the Brand Memo, which stated that agency “[g]uidance documents” without notice-and-comment rulemaking could not “create binding requirements that do not already exist by statute or regulation.”[61] DOJ guidance later that same year stated that DOJ “should not treat a party’s noncompliance with a guidance document as itself a violation of applicable statutes or regulations.”[62]
Early in the Biden Administration, this policy was reversed—with then-Attorney General Merrick Garland stating that guidance “may be entitled to deference or otherwise carry persuasive weight with respect to the meaning of the applicable legal requirements” in a particular case.[63] Given the second Trump Administration’s apparent focus on decreasing the reach of the administrative state, we expect that DOJ will again explore ways of limiting the use of sub-regulatory guidance to impose legal requirements on which FCA liability can be predicated.
Despite this shift, however, this administration is likely to use the FCA—as past administrations have—to enforce aspects of its policy agenda. In a notable early sign of this, President Trump’s January 21, 2025 Executive Order rescinding affirmative action obligations for federal government contractors requires that federal contracts and grants include a clause requiring contractors to agree that compliance “with applicable Federal anti-discrimination laws” is a term “material to the government’s payment decisions” for purposes of the FCA.[64]
It remains an open question whether DOJ under the second Trump Administration will place as much relative emphasis on voluntary dismissal of qui tam cases as DOJ did during Trump’s first term (after the release of the Granston Memo).[65] After the Granston Memo, voluntary dismissals spiked for the remainder of President Trump’s first term but immediately declined again when President Biden took office.
In outcome-oriented terms, one way to view voluntary dismissal under 31 U.S.C. § 3730(c)(2)(A) is as a business-friendly step that prioritizes the government’s view of a qui tam case’s prospects over the views of an incentivized relator. Voluntary dismissal by DOJ also could provide an answer to the concern expressed by Justices Thomas, Kavanaugh, and Barrett regarding the constitutionality of allowing unappointed relators to prosecute FCA cases in the government’s name. Indeed, voluntary dismissal permits DOJ to police unscrupulous relators and avoid a perception that it is letting itself be weaponized by private citizens. By the same token, voluntary dismissal also can help ensure that DOJ resources are appropriately conserved for the cases that most merit the government’s involvement—including cases without qui tam relators at all. Time will tell whether DOJ under the second Trump Administration uses its voluntary dismissal authority more frequently than has been the case over the last four years. But even that data point, by itself, is likely to be a poor predictor of DOJ’s overall level of aggressiveness when it comes to pursuing FCA cases, and more an indicator of how much stock it places in the available alternatives for doing so.
2. Anticipated Nominee to Head DOJ’s Civil Division
President Trump is expected to nominate Brett Shumate to serve as the Assistant Attorney General for the Civil Division. Shumate served as the Deputy Assistant Attorney General for the Civil Division’s Federal Programs Branch during the first Trump Administration. As the branch of the Civil Division that focuses “primarily o[n] defending suits that challenge actions of Government agencies and officers in which the plaintiffs seek injunctive or declaratory relief,” Federal Programs was a focal point of DOJ activity in the first Trump Administration. The appointment of a former Federal Program DAAG to run the entire Civil Division suggests that the administration is anticipating having to again devote significant resources to defending government actions in response to challenges.
3. Continued Emphasis on Cooperation and Remediation
The second half of 2024 witnessed a notable increase in the number of settlements that state that DOJ had credited settling parties for voluntary self-disclosures, remediation efforts, and/or cooperation with the government’s investigation. These factors are all relevant under the DOJ’s Guidelines for Taking Disclosure, Cooperation, and Remediation into Account in False Claims Act Matters.[66] Notably, while the frequency with which DOJ called out the fact of cooperation credit increased, details of exactly what steps earned the credit—and, critically, how much credit relative to the government’s claimed losses—remained few and far between.
In some instances, DOJ specified that it awarded “full credit” under its guidelines for voluntary disclosure in FCA cases. For instance, when a Northern Virginia hospital system agreed to pay $2.37 million to settle FCA allegations that it submitted claims for reimbursement to Medicaid that included documentation regarding sterilization and hysterectomy procedures that had been improperly modified, DOJ stated that the hospital system received “full credit” for disclosing the misconduct after undertaking an internal investigation, as well as for cooperating and taking “remedial actions.”[67]
DOJ policy caps cooperation credit so that the government does not receive “less than full compensation for the losses caused by the defendant’s misconduct (including the government’s damages, lost interest, costs of investigation, and relator share).”[68] In practice, that means a damages multiple above 1x—but how much above depends on the facts and circumstances. Without more details of the government’s claimed losses, the precise calculations and considerations are difficult to determine from the outside. It remains to be seen whether DOJ will introduce more transparency regarding its cooperation credit calculations into settlement agreements, of the sort that has become routine in criminal resolutions such as deferred prosecution agreements.
4. Administrative False Claims Act
In the final weeks of his term, President Biden signed into law a bill that authorized—with little fanfare—the Administrative False Claims Act (AFCA). The provisions establishing the AFCA were buried within defense spending legislation,[69] after attempts by Senator Chuck Grassley to pass the AFCA as a stand-alone bill failed in 2023 due to lack of House support.[70] The AFCA expands and replaces the Program Fraud Civil Remedies Act of 1986 (PFCRA), which provided an administrative remedy for false claims and statements with liability of less than $150,000.[71] As an administrative tool, the PFCRA allowed agencies to pursue small claims before an Administrative Law Judge (ALJ)—proceedings which lack the usual safeguards available to defendants charged in federal court, including the right to a trial by jury. The AFCA now authorizes agencies to challenge claims valued at up to $1 million before an ALJ and extends to false statements made even in the absence of a claim for payment. Agencies can seek civil penalties of up to $5,000 per claim, in addition to damages of up to twice the value of the claim. This significantly increases agencies’ ability to pursue and settle false claims allegations outside the federal judicial process.
The constitutionality of the AFCA may prove open to challenge. Last year, in SEC v. Jarkesy, the SEC attempted to impose civil penalties against an investment advisor for violating antifraud provisions in the federal securities laws.[72] The SEC elected to pursue an administrative remedy and adjudicate the matter before one of its ALJs, rather than in federal court where Jarkesy could have demanded a jury trial.[73] On appeal, the Supreme Court held that this violated the Seventh Amendment because the SEC’s claim against Jarkesy was “legal in nature,” which triggered the Seventh Amendment’s guarantee of a right to trial by jury.[74] Notably, the Supreme Court justified its decision, in part, by reasoning that the civil penalties sought were plainly punitive and therefore were the sort of common law remedy that could only be enforced in a court of law.[75] The SEC argued that the “public rights exception” should apply, which allows Congress to assign matters for decision to an agency when the issue historically could have been determined without judicial involvement.[76] But the Supreme Court disagreed, finding that the securities fraud action resembled a traditional legal claim modeled on common law fraud, which must be adjudicated by an Article III court.[77]
Although this ruling was limited to securities fraud, the similarities between the action brought in Jarkesy and those authorized under the AFCA cannot be ignored. Insofar as the AFCA is derivative of the FCA, it draws on common-law fraud and imposes remedies that are punitive in nature.[78] It remains to be seen whether defendants in AFCA actions are able to challenge the statute’s constitutionality using Jarkesy as a jumping-off point.
5. HHS-OIG Skilled Nursing Facilities and Nursing Facilities Compliance Program Guidance
One year after HHS-OIG’s release of its General Compliance Program Guidance, on November 20, 2024, HHS-OIG issued new industry segment-specific compliance program guidance for Skilled Nursing Facilities and Nursing Facilities (“Nursing Facility Guidance”).[79] The Nursing Facility Guidance has not been supplemented since 2008. In this iteration, HHS-OIG offers a deep dive on several compliance issues that could open nursing facilities to potential FCA liability. For example, the guidance cautions that sub-standard quality of care resulting from a lack of patient activities, staffing shortages, and poor medication management could lead to FCA violations.
This emphasis on quality of care as a vehicle for FCA enforcement actions underscores that regulators continue to take a more expansive view of what might qualify as an FCA violation under a “worthless services” theory—that is, the idea that the government receives nothing of value if it pays for services that are falsely represented as meeting relevant certification requirements. The Nursing Facility Guidance also details how AKS violations, referral relationships, more nuanced errors in Medicare and Medicaid billing, and data submission to managed plans can lead to the submission of false claims, signaling that the agency may continue to pursue aggressive FCA theories in these areas moving forward.
B. STATE LEGISLATIVE DEVELOPMENTS
There were no major developments with respect to state FCA legislation in the second half of 2024. HHS-OIG provides an incentive for states to enact false claims statutes in keeping with the federal FCA. If HHS OIG approves a state’s FCA, the state receives an increase of 10 percentage points in its share of any recoveries in cases involving Medicaid. The lists of “approved” state false claims statutes remains at 23 following the approval of Connecticut’s statute in February 2024; six states remain on the “not approved” list.[80] The other 21 states have either not enacted a state analogue or have not submitted their statutes for approval.
IV. CASE LAW DEVELOPMENTS
A. Federal District Court Holds That the Qui Tam Provisions Violate Article II of the Constitution
In September 2024, the Middle District of Florida held that the FCA’s qui tam provisions are unconstitutional because they violate the Appointments Clause. United States ex rel. Zafirov v. Fla. Med. Assocs., LLC, 2024 WL 4349242, at *1, *4 (M.D. Fla. Sept. 30, 2024). The issue arose from a qui tam suit filed by Clarissa Zafirov, who sued her employer and other defendants for violating the FCA by allegedly misrepresenting patients’ medical conditions to Medicare. Id. at *3. The government declined to intervene, and Zafirov continued litigating the action for five years. Id. at *4. Defendants moved for judgment on the pleadings, arguing that the FCA’s qui tam provisions violate Article II’s Appointments Clause, Take Care Clause, and Vesting Clause. Id. As to the Appointments Clause, defendants contended that the qui tam provisions violated the Appointments Clause because a relator is an improperly appointed officer of the United States. Id.
The Appointments Clause creates two different paths for appointment—one for “principal officers” and the other for “inferior officers.” Id. at *5. The appointment of inferior officers can be vested “in the President alone, in the Courts of Law, or in the Heads of Departments,” while principal officers that must be confirmed by the Senate. Id. (quotation omitted). An individual is considered an “officer of the United States” if she “exercis[es] significant authority pursuant to the laws of the United States,” and “occup[ies] a continuing position established by law.” Id. at *6 (quotations omitted).
In Zafirov, the court determined that FCA relators exercise “significant authority” because they possess civil enforcement authority on behalf of the United States through their “power to initiate an enforcement action in the name of the United States to vindicate a public right.” Id. (quotation omitted). The Court emphasized relators’ power to litigate appeals in FCA cases that can create binding precedent on the government. Id. at *7. As the Court noted, relators have the ability to initiate an action and litigate it in a way that binds the federal government while choosing “which defendants to sue,” “which theories to raise, which motions to file, and which evidence to obtain.” Id. at 11. The Court pointed to these as relators’ “powers.” Id. at 11. Lastly, the Court found that relators receive emoluments because they may receive a portion of the proceeds if their claims are successful. Id.
The court evaluated whether Article II included an exception for FCA relators to hold executive powers without complying with the process outlined in the Appointments Clause, but concluded that no such exception existed. Id. at *18. Because Zafirov was not appointed through the process outlined in the Appointments Clause, the court concluded that her lawsuit must be dismissed because “in Appointments Clause cases, invalidation is the remedy, which follows directly from the government actor’s lack of authority to take the challenged action in the first place.” Id. at 19 (internal quotation marks omitted). The United States promptly noticed an appeal to the Eleventh Circuit, where the case remains pending.
The Zafirov case has garnered much attention, not least of all for its apparent attempt to advance similar arguments that appeared in Justice Thomas’s dissenting opinion in United States ex rel. Polansky v. Executive Health Resources, Inc., 143 S. Ct. 1720 (2023). Those arguments were notable when they were published, both for their content and because they had not previously appeared in the majority opinions Justice Thomas has written on FCA issues in recent years (including Escobar, in which DOJ had declined to intervene). The arguments also gained support from Justices Kavanaugh and Barrett, who stated in a concurrence that the arguments were “substantial” and “should [be] consider[ed] . . . in an appropriate case.” Id. at 1737. (We covered the Polansky decision in more detail in Gibson Dunn’s 2023 Mid-Year False Claims Act Update.)
Those statements by Justices Thomas, Kavanaugh, and Barrett could mean that the Supreme Court will address the constitutionality of the qui tam provisions sooner than might otherwise be expected. On the other hand, a Supreme Court opinion on the constitutionality of the FCA’s qui tam provisions would arguably be the most significant decision by the Court on an FCA issue in the statute’s modern history, and other FCA issues that are highly consequential have taken years to find their way to the Court. (It took over two decades, for example, for the implied false certification theory to journey from a one-off Court of Federal Claims decision about the false “withholding of . . . information critical to the decision to pay” to the Supreme Court’s seminal decision on materiality in Escobar in 2016. See Ab-Tech Constr., Inc. v. United States, 31 Fed. Cl. 429 (1994).)
Regardless of the time horizon, we are likely to see continued momentum in efforts by qui tam relators to pursue cases—a reality foreshadowed by the record number of qui tam cases initiated in FY 2024, after the Polansky opinions were published. And defendants attempting to persuade courts to be skeptical of the qui tam provisions’ constitutionality will have to contend with longstanding precedents at the circuit level which hold that the provisions do not violate the Appointments Clause. See, e.g., Riley v. St. Luke’s Episcopal Hosp., 252 F.3d 749, 757 (5th Cir. 2001) (upholding the provisions on the grounds that relators do not meet “the constitutional definition of an ‘officer,’” which “encompasses, at a minimum, a continuing and formalized relationship of employment with the United States Government”); United States ex rel. Taxpayers Against Fraud v. Gen. Elec. Co., 41 F.3d 1032, 1041 (6th Cir. 1994) (similar).
B. The Second Circuit Adopts the “One-Purpose” Test for Pleading AKS Violations
In December 2024, the Second Circuit issued an opinion in United States ex rel. Camburn v. Novartis Pharms. Corp. that adopted the “at-least-one-purpose” rule for pleading the inducement element of an AKS-based FCA case. According to the Second Circuit, “a plaintiff adequately pleads an [AKS] violation when she states with the requisite particularity that at least one purpose of the alleged scheme was to induce fraudulent conduct.” 124 F.4th 129, 133 (2d Cir. 2024).
In Camburn, a former sales representative brought a qui tam lawsuit against the company alleging that it used speaker programs for its multiple sclerosis drug, Gilenya, as a vehicle to improperly remunerate physicians. Id. at 134. He alleged that Novartis orchestrated “sham” speaker events and engaged in other improper activities, all to incentivize physicians to improperly prescribe Gilenya. Id. at 135. The government declined to intervene. Id.
Camburn’s initial complaint was dismissed for failing to plead fraud with particularity under Rule 9(b). Id. Over successive amendments, Camburn incorporated testimony from 21 confidential witnesses spanning 21 states, supplementing his claims with additional factual allegations. Id. Nevertheless, the district court dismissed his Third Amended Complaint with prejudice, concluding that Camburn failed to adequately allege the existence of an AKS violation as a predicate for FCA liability. Id. Specifically, the court found that Camburn’s allegations lacked the requisite detail to support an inference that the company’s conduct was intended to induce fraudulent claims. Id.
The Second Circuit, however, partially reversed, concluding that Camburn’s specific allegations related to three categories of factual allegations—including dates, locations, and individuals involved—gave rise to a strong inference that one purpose of the conduct was to induce fraudulent claims, which was all that was needed to allege an AKS violation. Id. at 136-40. The court expressly rejected the notion that an FCA relator needs to allege “a cause-and-effect relationship (a quid pro quo) between the payments and the physicians’ prescribing habits” to plead an AKS violation. Id. at 137. With this ruling, the Second Circuit joined the First, Third, Fourth, Fifth, Seventh, Ninth, and Tenth Circuits in applying the “one-purpose” rule to the AKS. See Guilfoile v. Shields, 913 F.3d 178 (1st Cir. 2019); United States v. Greber, 760 F.2d 68 (3d Cir. 1985); United States v. Mallory, 988 F.3d 730 (4th Cir. 2021); United States v. Davis, 132 F.3d 1092 (5th Cir. 1998); United States v. Borrasi, 639 F.3d 774 (7th Cir. 2011); United States v. Kats, 871 F.2d 105 (9th Cir. 1989); United States v. McClatchey, 217 F.3d 823 (10th Cir. 2000).
Importantly, the question of what it means for false claims to “result[] from a violation” of the AKS, 42 U.S.C. § 1320a-7b(g) was not before the court. It remains to be seen whether the Second Circuit will adopt the stricter “but-for” causation standard advanced by the Sixth and Eighth Circuits, or the Third Circuit’s looser standard under which only some causal connection between kickback and false claim is required.
C. The Fifth Circuit Denies Relator a Share of Settlement Proceeds After Settlement with the Government
In a July opinion, the Fifth Circuit addressed whether a relator is entitled to a share of FCA settlement proceeds when the settlement does not resolve any of the claims brought by the relator. United States ex rel. Conyers, 108 F.4th 351 (5th Cir. 2024). The court concluded that a relator is entitled only to a share of the proceeds from the settlement of the specific claims they initiated, not from settlement of additional claims introduced by the government. Id. at 359.
The case originated when Bud Conyers filed a qui tam lawsuit alleging that a military contractor had violated the FCA. Id. at 353-54. The government intervened in the suit and added its own claims against the contractor that were focused on the alleged conduct of three employees and thus were distinct from Conyers’s original allegations. Id. at 354-55.
The parties eventually settled, with the contractor agreeing to pay approximately $13.7 million to resolve the government’s claims related to the three individuals, and with the agreement expressly reserving all other claims. Id. at 355. Conyers’ estate (the relator by then had passed away) sought a share of the settlement proceeds, arguing entitlement under the FCA. Id. The district court awarded Conyers’s estate approximately $1.1 million, finding some factual overlap between Conyers’s allegations and the government’s settled claims. Id.
On appeal, however, the Fifth Circuit reversed this decision, holding that under 31 U.S.C. § 3730(d)(1), a relator’s right to a share of the settlement proceeds is limited to the settlement of the specific claims that the relator brought. Id. at 356-61. The court stated that allowing relators to recover from settlements of claims they did not initiate would be inconsistent with the text of the FCA, which frames the relator’s share in a settlement scenario in terms of “claim[s].” Id. at 359. The court also invoked caselaw in the “alternate remedy” context which has held that that provision of the FCA only permits a relator to recover to the extent their claims “overlap[]” with the government’s claims. Id. at 358. The court further relied on the FCA’s purpose and structure as reflected in the reduction in a relator’s share that automatically comes with DOJ intervention, reasoning that it would run contrary to that framework to increase a relator’s share when the government opts not to pursue the claims the relator brings. Id. at 358-59.
Notably, the Fifth Circuit declined to decide whether Conyers would have been entitled to a share of the settlement proceeds if his claims had “factually overlapped” with those of the government, holding that the district court erred in determining there was sufficient overlap to merit that outcome even under such a standard. Id. at 359-60. At the core of the reversal decision was the fact that the three individuals whose conduct formed the basis of the settlement had never been mentioned in Conyers’s complaint. Id. at 359. Given that, one potential implication of the Conyers decision is an increased focus by relators on naming as many individuals as possible when making initial allegations, to maximize the chances that whatever claims-in-intervention the government may later bring bear a substantial enough relationship to the relator’s claims to justify the awarding of a relator’s share.
D. The Ninth Circuit Overrules Its Own Precedents on the First-to-File Bar, Holding the Bar Is Not Jurisdictional
The FCA’s first-to-file bar prevents anyone but the government from “interven[ing in] or bringing a related action based on the facts underlying [a] pending [FCA] action.” 31 U.S.C. § 3730(b)(5). In Stein v. Kaiser Found. Health Plan, Inc., the Ninth Circuit overturned its own precedents and held that the FCA’s first-to-file rule is not jurisdictional. 115 F.4th 1244, 1247 (9th Cir. 2024).
The district court had dismissed the case under the first-to-file bar because of its relation to already-pending actions against the same or other related entities. Id. at 1245. On appeal, a three-judge panel upheld the decision, but the full Ninth Circuit reversed after an en banc rehearing. Id. Citing recent Supreme Court decisions, the court ruled that a statutory bar is jurisdictional only if Congress explicitly says so. Id. at 1246 (citing Santos-Zacaria v. Garland, 598 U.S. 411, 416 (2023) (quoting Boechler, P.C. v. Comm’r, 596 U.S. 199, 203 (2022))). Because Section 3730(b)(5) does not use the term “jurisdiction” or include any other textual clue that points to jurisdiction, the court held that the FCA’s first-to-file rule is not jurisdictional. Id.
In so ruling, the Ninth Circuit joined five other circuits which had previously held that the FCA’s first-to-file rule is not jurisdictional. See United States ex rel. Bryant v. Cmty. Health Sys., Inc., 24 F.4th 1024, 1036 (6th Cir. 2022); In re Plavix Mktg., Sales Pracs. & Prods. Liab. Litig. (No. II), 974 F.3d 228, 232 (3d Cir. 2020); United States v. Millenium Lab’ys, Inc., 923 F.3d 240, 248-51 (1st Cir. 2019); United States ex rel. Hayes v. Allstate Ins. Co., 853 F.3d 80, 85-86 (2d Cir. 2017) (per curiam); United States ex rel. Heath v. AT&T, Inc., 791 F.3d 112, 119-21 (D.C. Cir. 2015).
The decision in Stein has at least three procedural implications. First, FCA defendants in the Ninth Circuit seeking to dismiss an FCA complaint based on the first-to-file rule can no longer make a Fed. R. Civ. P. 12(b)(1) motion to dismiss for lack of subject-matter jurisdiction, under which plaintiffs bear the burden of persuasion on the question of jurisdiction. Instead, defendants are left to rely on a Fed. R. Civ. P. 12(b)(6) motion to dismiss, which requires the moving party to show that the first-to-file bar requires dismissal of the FCA action. Second, before Stein, as an issue of subject-matter jurisdiction, a first-to-file challenge could be raised any time, and even sua sponte by the court. After Stein, defendants must raise the first-to-file challenge before the close of trial or risk waiving the defense altogether. Third, because the first-to-file rule is no longer considered jurisdictional after Stein, plaintiffs may have more flexibility to amend their complaints in response to a first-to-file pleading defense.
E. The Ninth Circuit Clarifies the Applicable Framework for FCA Retaliation Claims
The FCA prohibits retaliation against employees who report potential FCA violations. See 31 U.S.C. § 3730(h)(1). To prove retaliation under the FCA, an employee must have been engaging in protected conduct, the employer must have known that the employee was engaging in protected conduct, and the employer must have discriminated against the employee because of the protected conduct. In Mooney v. Fife, the Ninth Circuit (1) ruled that the McDonnell Douglas burden-shifting framework applies to FCA retaliation claims, and (2) clarified the “protected conduct” and “notice” requirements of a prima facie FCA retaliation claim. 118 F.4th 1081, 1090 (9th Cir. 2024).
The plaintiff in Mooney filed an FCA retaliation claim, alleging he was fired for reporting billing irregularities to his superiors, and claiming his employer used the confidentiality clause in his employment contract as a pretext for his termination. Id. at 1088. The district court rejected these claims and granted summary judgement for the defendant. Id. It concluded that the plaintiff’s reporting of irregularities could not have put his employer on notice of potentially protected conduct because the plaintiff’s job consisted of helping his employer ensure compliance with the law in the first instance. Id.
The Ninth Circuit reversed, concluding that the plaintiff had satisfied the three elements of a prima facie FCA retaliation claim, and noting that there were genuine issues of material fact as to plaintiff’s alleged pretextual firing. Id. at 1096-98. The Ninth Circuit joined a cohort of circuit courts that apply the McDonnell Douglas burden-shifting framework to FCA retaliation claims. Under this framework, once the employee has established a prima facie case of FCA retaliation, the burden shifts to the employer to produce a legitimate, non-retaliatory reason for the employee’s termination. Mooney, 118 F.4th at 1089. If the employer produces such a reason, the burden shifts back to the employee to show that the employer’s reason was pretextual. For purposes of pretext, it is irrelevant whether the employer’s proffered reason was objectively false; the only requirement is that the employer honestly believed the reasons for its actions, even if those reasons are foolish or trivial or even baseless. Id. at 1097 (citing Villiarimo v. Aloha Island Air, Inc., 281 F.3d 1054, 1063 (9th Cir. 2002)).
The Ninth Circuit also clarified the “protected conduct” and “notice” elements of an FCA retaliation claim. Regarding the “protected conduct” element, the Ninth Circuit stated that the applicable test, which contains both a subjective and an objective component, “does not set a high bar.” Mooney, 118 F.4th at 1092. The court narrowed the requirements applicable to both components. For the subjective component, the court noted that “the employee need not know for certain that the employer has committed fraud,” while for the objective component, the court noted that the only requirement is that “a reasonable employee in the same or similar circumstances might believe, that the employer is possibly committing fraud against the government.” Mooney, 118 F.4th at 1092 (citing Moore, 275 F.3d at 845). The Ninth Circuit also held that Hopper’s “investigating” requirement does not apply when the plaintiff alleges that he was discharged because of other efforts to stop one or more FCA violations. Id. at 1091. As to the “notice” requirement, the court refused to adopt a heightened pleading standard for employees with compliance duties. Id. at 1096. The Ninth Circuit reasoned that if compliance employees “were to have no protection from retaliation” under the FCA, “then fear of that retaliation could intimidate and discourage employees in such positions from trying to stop fraudulent billing practices.” Id. Instead, the court held that the “notice” element of an FCA retaliation claim only requires the employer to be aware of an employee’s efforts to stop one or more FCA violations. Id. (citing 31 U.S.C. § 3730(h)(1)).
F. The Third and Eleventh Circuits Apply the Public Disclosure Bar
1. The Third Circuit Finds the Bar Satisfied by Information in CMS’s Physician Payments Database
In United States ex rel. Stebbins v. Maraposa Surgical, Inc., 2024 WL 4947274, (3d Cir. Dec. 3, 2024), the Third Circuit Court of Appeals affirmed a district court’s dismissal of a qui tam action based on the public disclosure bar. In that case, a relator alleged that a medical office in Pennsylvania had violated the FCA by submitting reimbursement claims for arteriograms (medical imaging of arteries to identify and assess blockages) performed in its office. The relator claimed that because arteriograms could only be performed by a state licensed ambulatory surgery center, which the medical office was not, the medical office had fraudulently submitted reimbursements for its services.
After the medical center moved to dismiss the relator’s claims under Fed. R. Civ. P. 12(b)(6), the district court granted the motion. The relator appealed, and the Third Circuit affirmed, reasoning that because (1) the medical office’s reimbursement requests were publicly available on CMS’s payment database, (2) the medical office was not listed on the state’s online database of licensed ambulatory surgery centers, and (3) the state published the regulations on which the relator based his claims, the “essential elements” of the relator’s claims were “previously disclosed in publicly available databases.” Id. at *3. Because the relator was not a “original source of the information,” the Third Circuit determined that “anyone could [have] file[d] the same suit,” and, thus, the public disclosure bar applied. Id. at *2-3.
This case stands as another notable example of a court eschewing a narrow reading of the three relevant source categories listed in the public disclosure bar’s statutory language, in favor of an approach that broadly views information in public internet sources as disclosures sufficient to trigger the bar. The decision also avoids creating perverse incentives for providers to not report physician remuneration to CMS (if they feared that qui tam relators could use that public information to bring AKS allegations).
2. The Eleventh Circuit Rejects Relator’s Argument that His Legal Experience Made Him an “Original Source” Sufficient to Overcome the Public Disclosure Bar
In August, the Eleventh Circuit affirmed a district court’s dismissal of a qui tam action on the ground that the relator’s claims were barred by the FCA’s public disclosure provision. United States ex rel. Jacobs v. JP Morgan Chase Bank, N.A., 113 F.4th 1294 (11th Cir. 2024). There, the relator, a foreclosure attorney, alleged that JP Morgan Chase had violated the FCA by forging mortgage loan promissory notes and submitting false reimbursement claims to government-sponsored entities for loan servicing costs. JP Morgan Chase moved to dismiss the relator’s amended complaint, which the district court granted, concluding that the public disclosure bar precluded the relator’s claims.
The relator appealed, and the Eleventh Circuit affirmed. The Eleventh Circuit concluded that the public disclosure provision barred the relator’s suit because there were three online blogs that (1) were published before the relator initiated this suit, (2) met the FCA’s definition of “news media” because the blogs were “publicly available websites . . . intended to disseminate information,” and (3) contained substantially similar information to the relator’s claims. Id. at 1301-02. In reaching this determination, the Eleventh Circuit rejected the relator’s argument that his “experience from law practice” was enough to qualify him as an independent source of information. Id. at 1303. Because the relator did not provide any independent information to corroborate his claims, the Eleventh Circuit affirmed the dismissal of the qui tam action under the public disclosure bar.
V. CONCLUSION
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2025 False Claims Act Mid-Year Update.
[1] Press Release, U.S. Dep’t of Justice, Home Health Providers to Pay $4.5M to Resolve Alleged False Claims Act Liability for Providing Kickbacks to Assisted Living Facilities and Doctors (July 1, 2024), https://www.justice.gov/opa/pr/home-health-providers-pay-45m-resolve-alleged-false-claims-act-liability-providing-kickbacks.
[2] See Press Release, U.S. Atty’s Office for the Northern Dist. of Ohio, Rite Aid Corporation and Elixir Insurance Company Agree to Pay $101M to Resolve Allegations of Falsely Reporting Rebates (July 10, 2024), https://www.justice.gov/usao-ndoh/pr/rite-aid-corporation-and-elixir-insurance-company-agree-pay-101m-resolve-allegations.
[3] See Press Release, U.S. Atty’s Office for the Northern Dist. of N.Y., The Grand Health Care System and Twelve Affiliated Skilled Nursing Facilities to Pay $21.3 Million for Allegedly Providing and Billing for Fraudulent Rehabilitation Therapy Services (July 10, 2024), https://www.justice.gov/usao-ndny/pr/grand-health-care-system-and-twelve-affiliated-skilled-nursing-facilities-pay-213.
[4] See Press Release, U.S. Atty’s Office for the Northern Dist. of Ohio, Rite Aid Corporation and Affiliates Agree to Settle False Claims Act and Controlled Substance Act Allegations Related to Opioid Dispensing (July 11, 2024), https://www.justice.gov/usao-ndoh/pr/rite-aid-corporation-and-affiliates-agree-settle-false-claims-act-and-controlled; See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Rite Aid Corporation and Affiliates Agree to Settle False Claims Act and Controlled Substance Act Allegations Related to Opioid Dispensing (July 10, 2024),
[5] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Cal., Admera Health Agrees to Pay over $5 Million to Settle False Claims Act Allegations of Kickbacks to Third Party Marketers (July 24, 2024), https://www.justice.gov/usao-edca/pr/admera-health-agrees-pay-over-5-million-settle-false-claims-act-allegations-kickbacks.
See Press Release, U.S. Atty’s Office for the Western Dist. of Ky., Kindred and Related Entities Agree to Pay $19.428M to Settle Federal and State False Claims Act Lawsuits Alleging Ineligible Claims for Hospice Patients (July 18, 2024), https://www.justice.gov/usao-wdky/pr/kindred-and-related-entities-agree-pay-19428m-settle-federal-and-state-false-claims.
[7] See Press Release, U.S. Atty’s Office for the Dist. of Colo., DaVita to Pay Over $34M to Resolve Allegations of Illegal Kickbacks (July 18, 2024), https://www.justice.gov/usao-co/pr/davita-pay-over-34m-resolve-allegations-illegal-kickbacks.
[8] See Press Release, U.S. Atty’s Office for the Northern Dist. of Fl., Escambia County Pays $3.5 Million To Settle FCA Lawsuit (Aug. 1, 2024), https://www.justice.gov/usao-ndfl/pr/escambia-county-pays-35-million-settle-fca-lawsuit.
[9] See Press Release, U.S. Atty’s Office for the Southern Dist. Of Tex., NIRP and founder to pay nearly $9M to resolve alleged kickback referral violations (Aug. 20, 2024), https://www.justice.gov/usao-sdtx/pr/nirp-and-founder-pay-nearly-9m-resolve-alleged-kickback-referral-violations.
[10] See Press Release, U.S. Atty’s Office for the Western Dist. of Ky., Nationwide Home Healthcare and Hospice Provider to Pay $3.85M to Resolve False Claims Act Allegations (Aug. 20, 2024), https://www.justice.gov/opa/pr/nationwide-home-healthcare-and-hospice-provider-pay-385m-resolve-false-claims-act.
[11] See Press Release, United Seating and Mobility, LLC, D/B/A Numotion, Agrees to Pay $13,500,000 to Resolve Alleged False Claims for Custom Wheelchairs (Aug. 26, 2024), https://www.justice.gov/usao-az/pr/united-seating-and-mobility-llc-dba-numotion-agrees-pay-13500000-resolve-alleged-false.
[12] See Press Release, U.S. Atty’s Office for Dist. of Mo., U.S. Attorney Jesse Laslovich announces $10.8 million civil settlement with St. Peter’s Health over False Claims Act misconduct (Aug. 27, 2024), https://www.justice.gov/usao-mt/pr/us-attorney-jesse-laslovich-announces-108-million-civil-settlement-st-peters-health-over.
[13] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Pharmaceutical Company Pays $25 Million to Resolve Alleged False Claims Act Liability for Price-Fixing of Generic Drug (Sept. 4, 2024), https://www.justice.gov/usao-edpa/pr/pharmaceutical-company-pays-25-million-resolve-alleged-false-claims-act-liability.
[14] See Press Release, U.S. Atty’s Office for the Middle Dist. of Fl., Walgreens Agrees to Pay $106.8M to Resolve Allegations It Billed the Government for Prescriptions Never Dispensed (Sept. 13, 2024), https://www.justice.gov/usao-mdfl/pr/walgreens-agrees-pay-1068m-resolve-allegations-it-billed-government-prescriptions.
[15] See Press Release, U.S. Atty’s Office for the Dist. of S. Dakota, Surgical Hospital Agrees to Pay More Than $12.7M to Resolve Alleged False Claims Act Violations (Sept. 16, 2024), https://www.justice.gov/usao-sd/pr/south-dakota-surgical-hospital-agrees-pay-more-127m-resolve-alleged-false-claims-act.
[16] Press Release, U.S. Dep’t of Justice, Oak Street Health Agrees to Pay $60M to Resolve Alleged False Claims Act Liability for Paying Kickbacks to Insurance Agents in Medicare Advantage Patient Recruitment Scheme (Sept. 18, 2024), https://www.justice.gov/opa/pr/oak-street-health-agrees-pay-60m-resolve-alleged-false-claims-act-liability-paying-kickbacks.
[17] See Press Release, U.S. Atty’s Office for the Middle Dist. of Fl., Acadia Healthcare Company Inc. to Pay $19.85M to Settle Allegations Relating to Medically Unnecessary Inpatient Behavioral Health Services (Sept. 26, 2024), https://www.justice.gov/usao-mdfl/pr/acadia-healthcare-company-inc-pay-1985m-settle-allegations-relating-medically
[18] See Press Release, U.S. Atty’s Office for the East. Dist. of New York, Brooklyn-Based Home Health Care Agencies Settle Fraud Claims for $9.75 Million and Agree to Pay $7.5 Million in Wages and Benefits to Underpaid Aides (Sept. 30, 2024), https://www.justice.gov/usao-edny/pr/brooklyn-based-home-health-care-agencies-settle-fraud-claims-975-million-and-agree-pay.
[19] See Press Release, U.S. Atty’s Office for the Dist. of Mass., Brookline Hospital to Pay Up To $6.5 Million to Resolve False Claims Act Liability Concerning Kickback Allegations (Oct. 1, 2024), https://www.justice.gov/usao-ma/pr/brookline-hospital-pay-65-million-resolve-false-claims-act-liability-concerning-kickback.
[20] See Press Release, U.S. Atty’s Office for the Dist. of Colo., Precision Toxicology Agrees to Pay $27M to Resolve Allegations of Unnecessary Drug Testing and Illegal Remuneration to Physicians (Oct. 2, 2024), https://www.justice.gov/opa/pr/precision-toxicology-agrees-pay-27m-resolve-allegations-unnecessary-drug-testing-and-illegal.
[21] See Corporate Integrity Agreement Between the Office of Inspector General of The Department of Health and Human Services And Precision Toxicology, LLC D/B/A Precision Diagnostics, HHS-OIG (Oct. 22 2024), https://oig.hhs.gov/documents/cias/10036/Precision_Toxicology_LLC_DBA_Precision_Diagnostics_08222024.pdf.
[22] See Press Release, U.S. Atty’s Office for the Dist. of S.C., Operator of South Carolina Medicaid Call Center Agrees to Pay $11.3 Million to Resolve False Claims Act Liability; Two Former Employees Plead Guilty to Wire Fraud (Oct. 3, 2024), https://www.justice.gov/usao-sc/pr/operator-south-carolina-medicaid-call-center-agrees-pay-113-million-resolve-false-claims.
[23] See Press Release, U.S. Atty’s Office for the Northern Dist. of Tex, North Texas Medical Center Pays $14.2 Million to Resolve Potential False Claims Act Liability for Self-Reported Violations of Medicare Regs, Stark Law (Nov. 4, 2024), https://www.justice.gov/usao-ndtx/pr/north-texas-medical-center-pays-142-million-resolve-potential-false-claims-act.
[24] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Generic Pharmaceutical Company Pays $25 Million to Resolve False Claims Act Liability for Price-Fixing of Generic Drugs (Oct. 10, 2024), https://www.justice.gov/usao-edpa/pr/generic-pharmaceutical-company-pays-25-million-resolve-false-claims-act-liability; See Press Release, U.S. Atty’s Office for the Dist. of Mass., Teva Pharmaceuticals Agrees to Pay $425 Million to Resolve Kickback Allegations (Oct. 10, 2024), https://www.justice.gov/usao-ma/pr/teva-pharmaceuticals-agrees-pay-425-million-resolve-kickback-allegations.
[25] Press Release, U.S. Dep’t of Justice, Compound Ingredient Supplier Medisca Inc., to Pay $21.75M to Resolve Allegations of False and Inflated Average Wholesale Prices for Ingredients Used in Compounded Prescriptions (Nov. 1, 2024), https://www.justice.gov/opa/pr/compound-ingredient-supplier-medisca-inc-pay-2175m-resolve-allegations-false-and-inflated.
[26] See Press Release, U.S. Atty’s Office for the Dist. of Mass., QOL Medical and Its CEO Agree To Pay $47 Million for Allegedly Paying Kickbacks To Induce Claims for QOL’s Drug Sucraid (Nov. 15, 2024), https://www.justice.gov/usao-ma/pr/qol-medical-and-its-ceo-agree-pay-47-million-allegedly-paying-kickbacks-induce-claims.
[27] See Corporate Integrity Agreement Between the Office of Inspector General of The Department of Health and Human Services And QOl Medical, LLC, and Frederick E. Cooper, HHS-OIG (Nov. 1, 2024),
https://oig.hhs.gov/documents/cias/10135/QOL_Medical_LLC_and_Frederick_E_Cooper_11012024.pdf.
[28] See Press Release, U.S. Atty’s Office for the Dist. of Mass., Ethos Laboratories Agrees to Pay $6.5 Million to Resolve Allegations of Fraudulent Billing (Nov. 8, 2024), https://www.justice.gov/usao-ma/pr/ethos-laboratories-agrees-pay-65-million-resolve-allegations-fraudulent-billing.
[29] See Corporate Integrity Agreement Between the Office of Inspector General of The Department of Health and Human Services And Ethnos Holding Corp., D/B/A Ethos Laboratories, HHS-OIG (Nov. 1, 2024),
https://oig.hhs.gov/fraud/cia/agreements/Ethos_Holding_Corp_DBA_Ethos_Laboratories_11012024.pdf.
[30] See Press Release, U.S. Atty’s Office for the Dist. of Colo., UCHealth Agrees to Pay $23M to Resolve Allegations of Fraudulent Billing for Emergency Department Visits (Nov. 12, 2024), https://www.justice.gov/usao-co/pr/uchealth-agrees-pay-23m-resolve-allegations-fraudulent-billing-emergency-department.
[31] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Cal., Oroville Hospital to Pay $10.25 Million to Resolve Allegations of Kickbacks and False Billing (Dec. 12, 2024), https://www.justice.gov/usao-edca/pr/oroville-hospital-pay-1025-million-resolve-allegations-kickbacks-and-false-billing.
[32] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, California Hospital to Pay $10.25M to Resolve False Claims Allegations (Dec. 12, 2024),
https://www.justice.gov/opa/pr/california-hospital-pay-1025m-resolve-false-claims-allegations.
[33] See Press Release, U.S. Atty’s Office for the Dist. of Mass., Justice Department Announces Resolution of Criminal and Civil Investigations into McKinsey & Company’s Work with Purdue Pharma L.P.; Former McKinsey Senior Partner Charged with Obstruction of Justice (Dec. 13, 2024), https://www.justice.gov/usao-ma/pr/justice-department-announces-resolution-criminal-and-civil-investigations-mckinsey.
[34] See Press Release, U.S. Atty’s Office for the Western Dist. of N.Y., Medicare Advantage provider Independent Health to Pay up to $98m to Settle False Claims Act Suit (Dec. 20, 2024), https://www.justice.gov/usao-wdny/pr/medicare-advantage-provider-independent-health-pay-98m-settle-false-claims-act-suit.
[35] See Press Release, U.S. Atty’s Office for the Western Dist. of Ky., Sixteen Cardiology Practices to Pay a Total of $17.7M to Resolve False Claims Act Allegations Concerning Inflated Medicare Reimbursements (Dec. 20, 2024), https://www.justice.gov/usao-wdky/pr/sixteen-cardiology-practices-pay-total-177m-resolve-false-claims-act-allegations-0; Relator’s Complaint Under the False Claims Act, Walia et al. v. Michael et al., 1:18-cv-00510 (D.D.C. Mar. 5, 2018), ECF No. 1.
[36] See Integrity Agreement Between the Office of Inspector General of the Department Of Health and Human Services and Heart Clinic of Paris, P.A. and Dr. Arjumand Hashmi, HHS-OIG (Dec. 20, 2024), /https://oig.hhs.gov/documents/cias/10155/Heart_Clinic_of_Paris_PA_and_Dr_Arjumand_Hashmi_12202024.pdf.
[37] Press Release, U.S. Dep’t of Justice, Rapid Health Agrees to Pay $8.2M for Allegedly Billing Medicare for Over-the-Counter COVID-19 Tests That Were Not Provided to Beneficiaries (Dec. 20, 2024), https://www.justice.gov/opa/pr/rapid-health-agrees-pay-82m-allegedly-billing-medicare-over-counter-covid-19-tests-were-not
[38] See https://shorturl.at/yJsgI.
[39] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Tenn., Food City Agrees To Pay Over $8 Million To Settle False Claims Act Allegations Related To Opioid Dispensing (Dec. 23, 2024), https://www.justice.gov/usao-edtn/pr/food-city-agrees-pay-over-8-million-settle-false-claims-act-allegations-related-opioid.
[40] See Press Release, Southern California-Based Clinics, Laboratory, and Owners to Pay $15 Million to Settle Allegations of False Claims Arising from Kickbacks and Self-Referrals (Dec. 26, 2024), https://www.justice.gov/usao-cdca/pr/southern-california-based-clinics-laboratory-and-owners-pay-15-million-false-claims.
[41] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Avantor, Inc. Agrees to Pay $5.325 Million to Resolve Allegations of False Claims for Overcharging Federal Agencies and Allegations of DEA Violations and Lack of Compliance as to Listed Chemicals (July 31, 2024), https://www.justice.gov/usao-edpa/pr/avantor-inc-agrees-pay-5325-million-resolve-allegations-false-claims-overcharging.
[42] See Press Release, U.S. Atty’s Office for the Dist. of Mass., Raytheon Agrees to Pay Over $950 Million in Connection with Defective Pricing, Foreign Bribery and Export Control Schemes (Oct. 16, 2024), https://www.justice.gov/usao-ma/pr/raytheon-agrees-pay-over-950-million-connection-defective-pricing-foreign-bribery-and.
[43] See Press Release, U.S. Atty’s Office for the Dist. of Md., Paragon Systems Agrees to Pay $52M to Resolve False Claims Act Allegations Concerning Fraudulently Obtained Small Business Contracts and Kickbacks (Nov. 12, 2024), https://www.justice.gov/usao-md/pr/paragon-systems-agrees-pay-52m-resolve-false-claims-act-allegations-concerning.
[44] Press Release, U.S. Dep’t of Justice, Dell and Iron Bow Agree to Pay $4.3M to Resolve False Claims Act Allegations Relating to Submitting Non-Competitive Bids to the Army (Nov. 19, 2024), https://www.justice.gov/opa/pr/dell-and-iron-bow-agree-pay-43m-resolve-false-claims-act-allegations-relating-submitting-non.
[45] Press Release, U.S. Dep’t of Justice, Chemonics International Inc. to Pay $3.1M to Resolve Allegations of Fraudulent Billing Under Global Health Supply Chain Contract (Dec. 19, 2024), https://www.justice.gov/opa/pr/chemonics-international-inc-pay-31m-resolve-allegations-fraudulent-billing-under-global.
[46] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Ill., Virginia contractor to pay over $2.6M to settle allegations of falsely obtaining small business contracts (Jan. 7, 2025), https://www.justice.gov/usao-edva/pr/virginia-contractor-pay-over-26m-settle-allegations-falsely-obtaining-small-business.
[47] See Press Release, U.S. Department of Justice Office of Public Affairs, Five Point Enterprises Agrees to Pay the United States Over $2M for Submitting False Claims to VA for Post-9/11 GI Bill Education Benefits (Aug. 8, 2024), https://www.justice.gov/opa/pr/five-point-enterprises-agrees-pay-united-states-over-2m-submitting-false-claims-va-post-911.
[48] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Wisc., Two Brookfield, Wisconsin-Based Companies and Their Owners Pay Over $10 Million to Resolve Allegations that They Evaded Customs Duties, (August 8, 2024), https://www.justice.gov/usao-edwi/pr/two-brookfield-wisconsin-based-companies-and-their-owners-pay-over-10-million-resolve.
[49] See Press Release, U.S. Atty’s Office for the Southern Dist. of Fl., U.S. Attorney Lapointe Announces $7.6 Million Settlement of Civil False Claims Act Lawsuit Against Womenswear Company for Underpaying Customs Duties on Imported Women’s Apparel (Aug. 9, 2024), https://www.justice.gov/usao-sdfl/pr/us-attorney-lapointe-announces-76-million-settlement-civil-false-claims-act-lawsuit.
[50] See Press Release, U.S. Atty’s Office for the Central Dist. of Ca., City of Los Angeles Agrees to Pay $38.2 Million to Resolve False Claims Act Suit for Alleged Misuse of HUD Grant Funds (Aug. 26, 2024), https://www.justice.gov/usao-cdca/pr/city-los-angeles-agrees-pay-382-million-resolve-false-claims-act-suit-alleged-misuse.
[51] See Press Release, U.S. Atty’s Office for the Dist. of D.C., James B. Nutter & Company to Pay $2.4M for Allegedly Causing False Claims for Federal Mortgage Insurance (Sept. 23, 2024), https://www.justice.gov/usao-dc/pr/james-b-nutter-company-pay-24m-allegedly-causing-false-claims-federal-mortgage-insurance.
[52] https://www.justice.gov/opa/pr/gps-manufacturer-agrees-pay-26m-settle-false-claims-act-allegations-relating-improper.
[53] See Press Release, U.S. Atty’s Office for the Dist. of N.J., Atlantic County Health System Settles Matter Alleging It Received Improper Paycheck Protection Program Loan (Aug. 14, 2024), https://www.justice.gov/usao-nj/pr/atlantic-county-health-system-settles-matter-alleging-it-received-improper-paycheck.
[54] See Press Release, Brentwood-Based Dental Offices Company and Former Owners Pay $6.3 Million to Resolve False Claims Act Allegations Related to COVID Relief (Aug. 8, 2024), https://www.justice.gov/usao-cdca/pr/brentwood-based-dental-offices-company-and-former-owners-pay-63-million-resolve-false.
[55] See Press Release, U.S. Atty’s Office for the Middle Dist. of Fl., Travel Tourism Company Pays More Than $2 Million To Resolve Civil Claims Regarding Funds Obtained Under The Paycheck Protection Program (Sept. 17, 2024), https://www.justice.gov/usao-mdfl/pr/travel-tourism-company-pays-2-2-million-resolve-civil-claims-regarding-funds-obtained.
[56] See Press Release, U.S. Atty’s Office for the Southern Dist. of Ca., Del Mar Fairgrounds Agrees to Pay $5.6 Million to Settle Allegations Over Pandemic-Related Loan (Oct. 22, 2024), https://www.justice.gov/usao-sdca/pr/del-mar-fairgrounds-agrees-pay-56-million-settle-allegations-over-pandemic-related.
[57] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Wisc., Oak Creek company to pay over $2.3 million to resolve allegations it submitted false claims to obtain a Paycheck Protection Program Loan (Dec. 13, 2024), https://www.justice.gov/usao-edwi/pr/oak-creek-company-pay-over-23-million-resolve-allegations-it-submitted-false-claims.
[58] Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Deputy Attorney General Lisa O. Monaco Announces New Civil Cyber-Fraud Initiative (Oct. 6, 2021), https://www.justice.gov/opa/pr/deputy-attorney-general-lisa-o-monaco-announces-new-civil-cyber-fraud-initiative?utm_medium=email&utm_source=govdelivery.
[59] See Press Release, U.S. Dep’t of Justice, Three IRGC Cyber Actors Indicted for ‘Hack-and-Leak’ Operation Designed to Influence the 2024 U.S. Presidential Election (Sept. 27, 2024), https://www.justice.gov/opa/pr/three-irgc-cyber-actors-indicted-hack-and-leak-operation-designed-influence-2024-us.
[60] See Jonathan Greig, Major USAID contractor Chemonics says 263,000 affected by 2023 data breach, The Record (Dec. 5, 2024), https://therecord.media/chemonics-data-breach-usaid-contractor.
[61] U.S. Dep’t of Justice, Memorandum from Rachel Brand, Associate Attorney General (Nov. 16,
2017), https://www.justice.gov/opa/press-release/file/1012271/download.
[62] U.S. Dep’t of Justice, Justice Manual § 1-20.100 (Dec. 2018), https://web.archive.org/web/20190327044939/https://www.justice.gov/jm/1-20000-limitation-use-guidance-documents-litigation.
[63] See U.S. Dep’t of Justice, Memorandum from Merrick Garland, Attorney General (July 1, 2021), https://www.justice.gov/opa/file/1557606/dl?inline=.
[64] https://www.whitehouse.gov/presidential-actions/2025/01/ending-illegal-discrimination-and-restoring-merit-based-opportunity/.
[65] See U.S. Dep’t of Justice, Memorandum from Michael D. Granston, Director, Commercial
Litigation Branch, Fraud Section (Jan. 10, 2018),
https://drive.google.com/file/d/1PjNaQyopCs_KDWy8RL0QPAEIPTnv31ph/view.
[66] U.S. Dep’t of Justice, Justice Manual § 4-4.112 (Apr. 2018), https://www.justice.gov/jm/jm-4-4000-commercial-litigation.
[67] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Va., Virginia hospital system agrees to $2.37M False Claims settlement (Nov. 15, 2024), https://www.justice.gov/usao-edva/pr/virginia-hospital-system-agrees-237m-false-claims-settlement.
[68] Id.
[69] H.R. 5009, 118th Cong. § 5203 (2024) (enacted) (to be codified at 31 U.S.C. §§ 3801–12) (hereinafter AFCA).
[70] Administrative False Claims Act of 2023, S.R. 659, 118th Cong. (2023).
[71] AFCA § 5203(c).
[72] SEC v. Jarkesy, 603 U.S. 109 (2024).
[73] Id. at 109.
[74] Id. at 122–23.
[75] Id. at 123–25.
[76] Id. at 127.
[77] Id. at 134.
[78] See Universal Health Servs. v. United States ex rel. Escobar, 579 U.S. 176 (2016).
[79] See Office of Inspector General., U.S. Dep’t of Health & Hum. Servs., Nursing Facility Industry Segment-Specific Compliance Program Guidance (Nov. 2024) (hereinafter NF-ISPG), https://oig.hhs.gov/documents/compliance/10038/nursing-facility-icpg.pdf.
[80] State False Claims Act Reviews, Office of Inspector General, U.S. Dept’s of Health & Hum. Servs., https://oig.hhs.gov/fraud/state-false-claims-act-reviews/ (last visited Jan. 21, 2025).
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues and are available to assist in addressing any questions you may have regarding these issues. Please contact the Gibson Dunn lawyer with whom you usually work, the authors, or any leader or member of the firm’s False Claims Act/Qui Tam Defense practice group:
Washington, D.C.
Jonathan M. Phillips – Co-Chair (+1 202.887.3546, jphillips@gibsondunn.com)
Stuart F. Delery (+1 202.955.8515,sdelery@gibsondunn.com)
F. Joseph Warin (+1 202.887.3609, fwarin@gibsondunn.com)
Jake M. Shields (+1 202.955.8201, jmshields@gibsondunn.com)
Gustav W. Eyler (+1 202.955.8610, geyler@gibsondunn.com)
Lindsay M. Paulin (+1 202.887.3701, lpaulin@gibsondunn.com)
Geoffrey M. Sigler (+1 202.887.3752, gsigler@gibsondunn.com)
Joseph D. West (+1 202.955.8658, jwest@gibsondunn.com)
San Francisco
Winston Y. Chan – Co-Chair (+1 415.393.8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415.393.8391, cstevens@gibsondunn.com)
New York
Reed Brodsky (+1 212.351.5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212.351.3850, mdenerstein@gibsondunn.com)
Denver
John D.W. Partridge (+1 303.298.5931, jpartridge@gibsondunn.com)
Ryan T. Bergsieker (+1 303.298.5774, rbergsieker@gibsondunn.com)
Monica K. Loseman (+1 303.298.5784, mloseman@gibsondunn.com)
Dallas
Andrew LeGrand (+1 214.698.3405, alegrand@gibsondunn.com)
Los Angeles
James L. Zelenay Jr. (+1 213.229.7449, jzelenay@gibsondunn.com)
Nicola T. Hanna (+1 213.229.7269, nhanna@gibsondunn.com)
Jeremy S. Smith (+1 213.229.7973, jssmith@gibsondunn.com)
Deborah L. Stein (+1 213.229.7164, dstein@gibsondunn.com)
Dhananjay S. Manthripragada (+1 213.229.7366, dmanthripragada@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650.849.5395, bwagner@gibsondunn.com)
*Molly O’Neil, an associate in the Palo Alto office, is not admitted to practice law.
© 2025 Gibson, Dunn & Crutcher LLP. All rights reserved. For contact and other information, please visit us at www.gibsondunn.com.
Attorney Advertising: These materials were prepared for general informational purposes only based on information available at the time of publication and are not intended as, do not constitute, and should not be relied upon as, legal advice or a legal opinion on any specific facts or circumstances. Gibson Dunn (and its affiliates, attorneys, and employees) shall not have any liability in connection with any use of these materials. The sharing of these materials does not establish an attorney-client relationship with the recipient and should not be relied upon as an alternative for advice from qualified counsel. Please note that facts and circumstances may vary, and prior results do not guarantee a similar outcome.
This update summarizes recent enforcement activity, provides an overview of notable legislative and policy developments at the federal and state levels, and analyzes significant court decisions from the first half of the year.
I. Introduction
When the False Claims Act (“FCA”) is not making headlines on the Supreme Court’s docket, the flow of enforcement developments nonetheless remains constant. The first half of 2024 is a reminder that that flow can surge at any moment, bringing with it massive settlements for the government—over $1 billion over six months, in the case of 2024. Meanwhile, as the first half of 2024 also makes clear, there is never a dull moment when it comes to caselaw developments in the lower federal courts, and even when the U.S. Department of Justice (“DOJ”) is not being particularly vocal about its FCA enforcement priorities in speeches and publications, it often is taking steps in other enforcement contexts that have implications for the FCA.
In the first half of 2024, DOJ has continued its focus on FCA matters related to cybersecurity; has initiated pilot programs in criminal enforcement that have implications for qui tam whistleblower incentives; and has concluded settlements across a range of industries and legal theories, with the primacy of settlements in the healthcare industry continuing. Courts have grappled with issues such as FCA causation and the scienter required in FCA cases premised on violations of the Anti-Kickback Statute (“AKS”), and the Supreme Court granted certiorari in a case involving the definition of “claim” under the FCA.
Below, we summarize recent enforcement activity, then provide an overview of notable legislative and policy developments at the federal and state levels, and finally analyze significant court decisions from the first half of the year. Gibson Dunn’s recent publications regarding the FCA may be found on our website, including in-depth discussions of the FCA’s framework and operation, industry-specific presentations, and practical guidance to help companies navigate the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
II. Noteworthy DOJ Enforcement Activity During the First Half of 2024
2024 has been a notable half-year for FCA settlements by DOJ: during the first six months of the year, the government announced resolutions totaling over $1 billion.[1] That dollar figure is the highest for the first half of a calendar year—by a significant margin—in recent memory. It also includes two nine-figure settlements, whereas the first half of 2023 included none and the first half of 2022 included only one. While both of those nine-figure settlements grant DOJ claims in the bankruptcy cases of the settling counterparties and the government thus stands to recover significantly less than the settlements’ face values, the fact of resolutions valued at those figures remains a significant development.
Below, we summarize the most notable settlements and judgments from the first half of this year, organized by industry and focused on key theories of liability at issue in the resolutions. As usual, FCA recoveries in the healthcare and life sciences industries dominated enforcement activity during the first half of the year in terms of the number and value of settlements. DOJ, however, also announced notable resolutions in the government contracting and procurement space, described below.
A. Healthcare and Life Science Industries
As usual, the vast majority of FCA recoveries in the first half of 2024 involved entities and individuals in the healthcare and life sciences industries.
- On January 4, a healthcare facility operator in Delaware agreed to pay $42.5 million to resolve allegations that the company provided ancillary service providers—including nurse practitioners and physician assistants—to assist with patients as an inducement to non-employee doctors to refer patients to the company’s hospitals. The complaint alleged that these arrangements violated the AKS and the Stark Law. The allegations underlying the settlement agreement stemmed from a qui tam suit brought by the company’s former chief compliance officer, who will receive an unspecified portion of the recovery.[2]
- On January 4, a Florida non-profit agreed to pay approximately $19.5 million to resolve allegations that it billed federal healthcare programs for items and services used in clinical trial research that it should have billed to non-government sponsors. The organization itself initiated an independent investigation into the alleged behavior and disclosed its findings to the government. The federal government will receive $18.2 million from the settlement, and Florida Medicaid will receive $1.3 million.[3]
- On January 4, a Memphis hospital system agreed to pay $7.25 million to resolve claims that it submitted false claims to Medicare that arose out of improper financial arrangements. Specifically, the government alleged the hospital system had a multi-agreement relationship with a medical clinic, and the hospital system used these various business contracts as a vehicle to pay kickbacks to the clinic to induce it to refer Medicare beneficiaries to the hospital system. The suit resolves a qui tam suit brought by a former president of a hospital within the system and a medical school dean, who will each receive an unspecified portion of the settlement.[4]
- On January 5, an Arizona home health agency agreed to pay nearly $10 million to resolve allegations that it submitted false claims to a healthcare program serving Department of Energy employees and contractors with occupational illness. The government alleged that the agency billed the program for nursing and care services when its employees were not physically present in the patients’ homes. It further alleged that the agency’s “friends and family” program violated the AKS by paying cash and in-kind payments for food, travel, and other expenses in exchange for patient referrals. The agency made a voluntary disclosure to the government regarding its friends and family program and in-kind remuneration, and the settlement agreement acknowledges the agency’s cooperation in this regard. The settlement resolves a qui tam suit brought by a former Corporate Administrator and Director of Human Resource Administration and Management at the agency and its predecessor, who will receive approximately $1.7 million from the settlement.[5]
- On January 10, a New Jersey clinical laboratory and its CEO agreed to pay $13.2 million to resolve allegations that it billed federal healthcare programs for laboratory tests procured through illegal kickbacks. The government alleged that the laboratory obtained referrals through five different kinds of kickbacks, including: (1) commissions paid based on volume and value of referrals to the laboratory through independent contractors; (2) payments disguised as management services organization fees that were actually incentives for laboratory referrals; (3) payments to healthcare providers disguised as consulting or medical director fees in exchange for ordering lab tests; (4) payments to substance abuse recover centers to induce referrals for lab testing; and (5) specimen collection fees to healthcare providers to induce referrals to the laboratory. In addition, the government alleged that the laboratory and CEO submitted claims for tests that were not medically necessary or not otherwise covered by Medicare and Medicaid.[6]
- On January 11, a long-term care hospital agreed to an ability-to-pay settlement requiring it to pay over $18.6 million plus 4.5% interest per year to resolve allegations that the hospital impermissibly claimed excessive cost outlier payments from Medicare. Specifically, the government alleged that the hospital manipulated the cost outlier payment system for supplemental reimbursement by increasing its charges in excess of its costs and beyond what the hospital would be able to repay once Medicare cost reports were reconciled to its charges. In addition to the FCA claim, the settlement involved a $12 million penalty resolving Federal Debt Collection Procedures Act allegations against certain hospital investors for their role in the hospital’s alleged fraudulent transfer of money without equivalence value exchange to its investment management company.[7]
- On January 17, a healthcare company and its owners consented to a $2 million judgment, admitting to FCA violations for using medical staff to submit claims for medically unnecessary care to federal healthcare programs. The government alleged that the company hired vulnerable or inexperienced medical staff and then pressured those staff members to provide unnecessary care, and to submit the claims for that care to federal payors. The government further alleged that the company falsified information to obtain Paycheck Protection Program (PPP) loan forgiveness. The consent agreement resolves allegations under the FCA, AKS, and Controlled Substances Act.[8]
- On January 23, a Philadelphia pharmacy and its current and former owners agreed jointly to pay approximately $3.9 million to resolve allegations that they billed Medicare and Medicaid for medications that were never dispensed from January 2018 through September 2020. The government also alleged that in some cases the pharmacy dispensed low-cost formulations to beneficiaries but billed Medicare for the high-cost versions of the formulations. The pharmacy and its principal pharmacist entered into an integrity agreement requiring them to undertake significant compliance obligations and conduct third-party audits of their Medicare claims and drug inventory through an Independent Review Organization.[9]
- On January 26, a group of durable medical equipment companies agreed to pay $2.1 million to resolve allegations that they submitted false claims to federal healthcare programs by selling used hospital beds as if they were new. The government also alleged the companies upcoded support products and mischaracterized non-reimbursable travel time as repair time in claims for payment made to federal programs and contractors. This settlement resolved a related qui tam suit brought by a former employee, who will receive an undisclosed portion of the settlement amount.[10]
- On January 30, a drug rehabilitation facility and a clinical laboratory agreed to resolve liability for submitting false claims for urine drug testing services to the federal Medicare and Kentucky state Medicaid programs by paying $2.2 million and $4.9 million respectively. The government alleged that the drug rehabilitation facility used the same complex panel of urine drug tests for all patients on a weekly basis, despite the results often not being used for diagnosis or treatment and without considering whether individual patients needed the panel. The clinical laboratory performed the urine tests and billed them to federal and state healthcare programs despite knowing that the tests were not typically used for diagnosis or treatment and also performed additional urine drug screens without proper medical orders requesting the screens. As a part of the settlement, the drug rehabilitation facility entered into a corporate integrity agreement with HHS-OIG, which requires the facility to appoint a compliance officer and retain an independent expert for its compliance program. The clinical laboratory’s share of the settlement will require it to cease operations and pay the United States 100% of the net proceeds of the sale of its assets, along with 70% of reimbursements from healthcare payors for one year and any employee retention tax credit funds received. The settlement resolves a related qui tam suit, with the relator receiving an undisclosed portion of the recovery.[11]
- On February 7, a Pennsylvania multi-hospital system agreed to pay $11.7 million to resolve allegations that it submitted claims to Medicare for services relating to annual wellness visits. The hospital system voluntarily disclosed that it submitted claims that were not supported by the medical record between December 2015 and November 2022. Following its self-disclosure, the government noted, the hospital system took corrective action, although in resolving the case the government did not specify what that action was.[12]
- On February 14, a medical equipment company that rented non-invasive ventilators agreed to pay $25.5 million to resolve allegations that it continued to bill federal health care programs after patients ceased using their devices. Additionally, DOJ alleged that the company failed to confirm that the devices it rented were medically necessary, impermissibly waived coinsurance payments to get more patients to rent their equipment, and paid kickbacks to induce Medicare beneficiaries to rent its equipment. The company admitted it received reimbursement for claims it submitted to federal healthcare programs that did not comply with Medicare billing guidelines. This resolved a related qui tam suit for which the relator will receive an unspecified portion of the proceeds.[13]
- On February 16, a Kentucky toxicology lab and its owner agreed to a nearly $5.6 million judgment for violating the FCA by charging court‑ordered urine tests to Medicare and Medicaid, even though the tests were not medically necessary. The lab’s compliance officer also agreed to a $4.87 million judgment against her for a related scheme in which she solicited urine drug tests from non-medical homeless shelters and charged those tests to Medicare and Medicaid. Both the owner and the compliance officer received prison sentences of 46 months and six months respectively for related criminal charges. Furthermore, the lab, the owner, and the compliance officer will be excluded from federal health care program participation for 20 years. The consent agreements resolve a qui tam suit for which the relator will receive an unspecified portion of the proceeds.[14]
- On February 28, a pharmaceutical manufacturer, DOJ and an ad hoc group of first lien creditors reached a comprehensive settlement of all federal government claims against the manufacturer. The settlement included resolution of FCA claims asserted by DOJ, which were resolved by granting DOJ a $475.6 million general unsecured claim in the manufacturer’s chapter 11 bankruptcy cases. DOJ alleged that the company marketed its opioid drug to providers the company knew prescribed the drug for non-medically accepted indications, and that the company incentivized such targeting through sales goals, employee incentive compensation plans, and employee performance reviews. In resolution of a parallel criminal investigation, the comprehensive settlement also required a debtor affiliate of the manufacturer to plead guilty to a misdemeanor violation of the Food, Drug and Cosmetic Act (“FDCA”) based on allegations that it introduced misbranded drugs into interstate commerce. Altogether the comprehensive settlement encompassed approximately $8 billion of alleged claims asserted by the IRS, the civil and criminal branches of DOJ, and several federal healthcare agencies. Under the terms of the comprehensive settlement, the company made a single $200 million payment in satisfaction of all the government’s claims upon the effective date of its chapter 11 plan of reorganization in April 2024, and the settlement allowed the company’s pharmaceutical business to emerge under such plan.[15] Gibson Dunn represented the first lien ad hoc group, which negotiated the foregoing economic settlement with DOJ, and was intimately involved in all aspects of this comprehensive resolution and its implementation.
- On February 28, a Georgia laboratory and its owner agreed to pay $14.3 million to partially resolve allegations that it submitted false claims to government healthcare programs. In particular, the government alleged that the owner paid independent contractors to recommend that senior living communities order expensive and unnecessary respiratory pathogen panels (RPPs), rather than the COVID-19 tests that the communities initially requested. The contractors also, with the owner’s alleged knowledge, performed COVID-19 tests in senior living communities, but then arranged for the laboratory to submit claims to federal health plans using sham Medicaid diagnosis codes that did not reflect the medical conditions of those receiving the tests. The contractors also allegedly forged physician signatures on RPP order forms. The owner, along with four other people, pleaded guilty to criminal charges connected to the scheme. The federal government will receive $13.9 million from the civil settlement, and Georgia will receive $400,000. The settlement also resolves a qui tam suit for which the relator will receive $2.86 million.[16]
- On March 6, a hospital system in New York agreed to pay $17.3 million to resolve allegations that it paid unlawful kickbacks to doctors at the hospital’s chemotherapy infusion center. The government alleged that the hospital entered into contracts with the physicians that linked the physicians’ compensation to the number of referrals made for services at the chemotherapy center. The settlement agreement also resolves claims that the physicians failed to adequately supervise these services as required by Medicare and Medicaid regulations, in addition to claims under New York’s state FCA statute. The hospital voluntarily disclosed the information to the United States.[17]
- On March 6, a generic pharmaceutical manufacturer agreed to pay $2 million to resolve allegations that it submitted false claims to TRICARE, the VA, the Federal Employees Health Benefits Program, and the Department of Labor Office of Works Compensation Programs. The government alleged that the company sold adulterated pharmaceuticals after failing to follow controls required by manufacturing regulations, which resulted in the submission of false claims. This settlement resolved the civil liability component of a criminal investigation related to the introduction of adulterated drugs into interstate commerce. The company also entered into a plea agreement to resolve the related criminal indictment, pursuant to which it agreed to a three-year deferred prosecution agreement and to pay an additional $1.5 million fine.[18]
- On March 20, two former Philadelphia-based pharmacy employees agreed to pay over $4.1 million to resolve liability under the FCA and Controlled Substances Act for illegally dispensing and distributing controlled substances and engaging in fraudulent billing. Specifically, the government alleged that the former employees dispensed opioids and other “cocktail” drugs in extreme doses and combinations under highly suspicious circumstances, including excessive cash payments and clearly forged prescriptions. The employees also engaged in a scheme using a “BBDF” (“Bill But Don’t Fill”) code to falsely claim to Medicare and other insurers that drugs had been dispensed to patients. The former employees also pled guilty to related criminal charges and were sentenced to several months imprisonment along with receiving permanent bans on dispensing controlled substances. The civil settlement and criminal convictions marked the end of a multi-year investigation into related fraudulent activity at the pharmacy, including activities by its owner and other employees.[19]
- On March 25, a clinical laboratory and its owners agreed to pay approximately $13.6 million and to be excluded from federal health care programs for 15 years to resolve allegations that they submitted Medicare claims for tests that were neither medically necessary nor ordered by healthcare providers. Specifically, the government alleged that the laboratory performed and submitted claims for medically unnecessary urinary tract infection panel of tests by PCR when physicians only ordered a less extensive urinalysis tests as part of an illegal kickback scheme. The laboratory allegedly did so because Medicare reimbursements for the PCR tests were significantly higher than reimbursements for the tests the physicians had ordered. This settlement resolved a related qui tam action brought by a physician who owned health care facilities and served patients for whom the laboratory ran tests. The physician relator will receive approximately $2.3 million of the settlement amount.[20]
- On March 25, a healthcare staffing company agreed to pay approximately $9.3 million to resolve FCA and criminal liability regarding its visa sponsorship program. Specifically, the government alleged the staffing company submitted false visa immigrant applications, provided false job placement letters, and made false statements to government officials while recruiting healthcare professionals into the United States. Along with undertaking extensive remedial efforts in its compliance, the company also pledged an additional $8 million to healthcare projects in an effort to address harms caused by its prior practices. The pledge will be distributed to various NGOs and non-profits involved with ethical recruitment, strengthening healthcare access and infrastructure in certain developing countries and in rural/underserved U.S. communities.[21]
- On March 27, a Georgia teleradiology company and its CEO agreed to pay $3.1 million to settle liability for violations of the FCA and comparable state laws for fraudulently billing federal health care programs. The government alleged that the company’s U.S.-based radiologists failed to adequately review interpretation reports prepared by overseas contractors who were not permitted to practice medicine in the U.S. or bill U.S. federal healthcare programs. The company also misrepresented which medical professionals actually rendered radiology services, and improperly sought reimbursement for services provided by medical professionals outside of the United States. Approximately $2.68 million of the settlement will be paid to the U.S., and the remaining $420,000 will be distributed to various states. The settlement additionally resolves a qui tam suit, but it was not disclosed whether the relators would receive a share of the settlement.[22]
- On April 2, a Texas oncology practice and diagnostic reference laboratory agreed to pay approximately $4 million to resolve allegations that they violated the AKS. The government alleged that the laboratory offered illegal kickbacks in exchange for bone marrow biopsy exams, which induced physicians to order the tests. Furthermore, the government contended that one of the practice’s physicians billed federal and state healthcare programs for medically unnecessary tests and services. This settlement resolved a qui tam suit brought by a former physician at the practice who will receive an unspecified amount. The oncology practice also entered into an integrity agreement for a period of three-years as part of the settlement.[23]
- On April 9, a California-based nursing home chain agreed to pay approximately $7 million to resolve allegations that it submitted claims to Medicare and Medicaid for reimbursement for skilled care that it did not actually provide. The government alleged that the company misused a COVID-19 emergency waiver that removed the three-day hospital stay requirement to receive reimbursement for skilled care for nursing home residents. The company allegedly submitted claims for skilled care reimbursement when residents at the home were merely exposed to COVID-19, rather than infected, and as a result did not actually receive skilled care in the nursing home. The company will pay the federal government approximately $6.8 million and the state of California approximately $242,000, plus interest. The company will also enter a Corporate Integrity Agreement (CIA) with the Department of Health and Human Services, Office of Inspector General (HHS-OIG). The settlement resolves a qui tam suit for which the relators will receive approximately $1.2 million, plus interest.[24]
- On April 24, an Atlanta-based company agreed to pay $2.7 million to resolve allegations that it violated the False Claims Act by failing to implement adequate cybersecurity measures to protect health information obtained through a contract with the Pennsylvania Department of Health to provide staffing for COVID-19 contact tracing. The government alleged that the company transmitted confidential and/or personally identifiable information in unencrypted emails, that it stored and transmitted information through Google files that were not password protected, and that staff used shared passwords to access the information. The government also alleged that the company received complaints for at least five months before the company started remediating the issue. The settlement resolves a qui tam lawsuit brought by a former staff member at the company, who will receive a $499,500 share of the lawsuit.[25]
- On April 25, a healthcare management company and its subsidiaries agreed to pay $4.2 million to resolve allegations that it knowingly submitted false Medicare claims. In particular, the government alleged that the company retained overpayments for hospice care claims when the patients were not terminally ill and therefore ineligible for hospice care. This settlement resolved a qui tam suit brought by a former employee, who received $672,000 of the settlement.[26]
- On May 6, the owner and operator of multiple medical diagnostic and laboratory-related LLCs agreed to pay $27 million to resolve allegations that he and his companies conspired to violate the FCA by submitting false claims to federal healthcare programs for medically unnecessary cancer genomic tests (CGx) procured through illegal kickbacks. In particular, the government alleged that he and his companies conspired with telemarketers to solicit Medicare beneficiaries for CGx tests and conspired with telemedicine providers to prescribe medically unnecessary CGx tests. The government further claimed that he and his companies conspired with reference laboratories that would perform the CGx tests, and with billing laboratories and a hospital to submit claims to Medicare and Medicaid. The Floridian owner and operator previously pled guilty to criminal healthcare fraud related to this same conduct in 2022. As part of this settlement agreement, his companies agreed to be excluded from all federal health care programs. This settlement resolves three related qui tam actions, including one action brought by a minority owner of one of the LLCs, who will receive approximately $4.7 million of the settlement amount.[27] The portion of the settlement that the other relators will receive is not specified.
- On May 8, a Michigan healthcare practice agreed to pay approximately $2 million to resolve allegations that it submitted claims for improperly supervised medical care to Medicare and Medicaid. In particular, the government alleged that the company submitted claims to Medicare and Medicaid for procedures performed by physician assistants in nursing home facilities without the required doctor supervision. The state of Michigan will receive approximately $66,000 from the settlement.[28]
- On May 16, a Massachusetts hospital agreed to pay $24.3 million to resolve allegations that it submitted claims to Medicare for medical treatments that did not comply with Medicare rules about evaluating patient suitability for the prescribed medical treatment. Specifically, the government alleged that the hospital knowingly submitted claims for transcatheter aortic valve replacement (TAVR) procedures without the required number of physicians either examining the patient or documenting their judgment regarding the patient’s suitability for the procedure. As part of the settlement, the hospital will enter into a five-year CIA with HHS-OIG under which an Independent Review Organization will annually review the hospital’s Medicare charges. Because the hospital voluntarily assisted the government during its investigation, the hospital received cooperation credit pursuant to DOJ guidelines. The settlement resolves a qui tam suit for which the relator will receive approximately $4.36 million.[29]
- On May 17, a medical clinic agreed to pay $7.6 million to resolve allegations that it violated the FCA in connection with three federal grant awards for its research. In particular, the government alleged the clinic failed to disclose that the Principal Investigator on each grant was an employee with pending or active grants from foreign institutions who supported that employee’s research and obligated the employee’s time, which violated the National Institute of Health (NIH)’s transparency requirements. The settlement also resolved allegations that the clinic impermissibly allowed its employees to share passwords for access to the NIH grant reporting platform, which resulted in other employees making false submissions in the name of the Principal Investigator without their knowledge. The HHS-OIG, FBI, and two assistant U.S. attorneys collaborated with the U.S. Attorney’s Office for the Northern District of Ohio to resolve these allegations. The clinic agreed to implement a Corrective Action Plan, and NIH imposed specific award conditions for future grants for at least a one-year period or until completion of the Corrective Action Plan.[30]
- On May 20, two New York not-for-profit corporations agreed to pay approximately $10 million to resolve allegations that they submitted false claims to Medicaid for certain long-term care services. The companies administered a Managed Long Term Care Plan (“MLTCP”) for Medicaid beneficiaries, under which they arranged for health and long-term care services and were reimbursed by Medicaid through per-member payments on a monthly basis. As part of the settlement, the companies admitted to collecting payments for the services under the MLTCP that they did not provide or did not adequately document the provision of. The settlement also resolves a qui tam suit brought by a relator. The portion of the settlement that relator will receive is not specified.[31]
- On May 22, a medical device manufacturer and two senior executives agreed to pay $12 million to resolve allegations that they violated the False Claims Act by paying kickbacks to spine surgeons to induce the surgeons to use the company’s spinal devices. According to the government’s allegations, the company provided improper renumeration to spinal surgeons in the form of consulting and other fees, registry payments, performance shares, and travel and lavish dinners. The settlement also resolves a qui tam action brought by a former regional sales director for the company, who will receive an approximately $2.2 million share of the recovery.[32]
- On May 28, three affiliated healthcare companies operating in Florida, Minnesota, and Wisconsin agreed to pay approximately $14.9 million to resolve allegations that they improperly billed Medicare, Medicaid, and TRICARE by knowingly submitting claims for two Evaluation and Management codes that did not support the level of service that the companies actually provided. Under the settlement, the federal government will receive approximately $13.8 million, and the state governments of Florida and Minnesota will receive approximately $1 million. The company must also enter into a five-year CIA with HHS-OIG, which will require the company to establish and maintain a compliance program and submit to an Independent Review Organization’s review of its Medicare claims to ensure that they are medically necessary. The settlement also resolves a qui tam suit for which the relator will receive approximately $2.8 million.[33]
- On June 6, defendants in a New-York ophthalmologist practice agreed to pay approximately $2.5 million to resolve claims that, over a three-year period, they billed federal healthcare programs for medically unnecessary tests and procedures, and services that could not have been performed because the doctor was not in the office at the time the services were purportedly rendered. The government further alleged that the scheme exploited residents in Brooklyn and Queens, many of whom were non-native English speakers or elderly. The settlement agreement resolves two qui tam actions but does not specify the relators’ shares of the recovery.[34]
- On June 11, a Chicago-based nurse practitioner group and its former owners agreed to pay approximately $2 million to resolve allegations that it submitted false claims to Medicare and Medicaid. The government alleged that the company and its owners developed patient charting software that generated false, upcoded claims for Medicare and Medicaid. According to the government’s allegations, the company and its owners required its nurse practitioners to use the software, despite knowing that it resulted in fraudulently upcoded claims being submitted to and paid by Medicare and Medicaid. The settlement also resolves a qui tam lawsuit brought by a former employee, which receive approximately $358,647 as part of the settlement.[35]
- On June 24, medical centers and a medical college in Texas agreed to pay $15 million to resolve claims they billed for concurrent heart surgeries in violation of Medicare teaching physician and informed consent regulations. According to the government’s allegations, three heart surgeons at the medical center ran a regular practice of running two operating rooms at once, delegating key aspects of the surgeries to unqualified medical assistants. The $15 million recovery is the largest settlement to date involving concurrent surgeries. Under the settlement, the qui tam relator will receive approximately $3.1 million.[36]
B. Government Contracting and Procurement
- On January 19, an oil and gas company agreed to pay $34.6 million to resolve allegations it knowingly underpaid royalties owed on oil and gas produced from federal lands. Specifically, the government alleged that the company submitted royalty payments to the federal government based on estimates and subsequently failed to make follow-up payments based on actual volumes and values as required by its agreements with the government. The company received credit under the settlement for cooperation by assisting with the determination of losses.[37]
- On January 30, a technology company agreed to pay $5 million to resolve allegations that it falsely overstated cost and pricing data in a subcontract proposal to the U.S. Army. Specifically, the government alleged that the company overstated its costs to a primary contractor who was negotiating with the Army, and that the primary contractor then relied on those estimated costs when negotiating its contract, leading to significant overcharges. The settlement resolves a related qui tam suit for which the relator will receive $900,000.[38]
- On January 30, a Virginia-based consulting agency and its parent company agreed to pay $3.9 million to resolve allegations that it made false statements about its status as a women-owned small business (WOSB) to obtain a Defense Health Agency contract regarding providing doctors to an Air Force treatment facility that had been set aside for WOSBs. In particular, the government alleged that the consulting company forfeited its WOSBs status when it failed to update its size certifications post-acquisition as required, and when asked by the government’s contracting official. The company was awarded the contract based on the allegedly false representation when it would not have been eligible had it provided correct information. This settlement resolved a qui tam action brought by an entity healthcare and support services provider, which purportedly discovered the misrepresentations through a report it developed to analyze Defense Health Agency contracts.[39] The settlement amount reflects cooperation from the companies during the government’s investigation.[40]
- On March 12, an information and advisory services company agreed to pay $37 million to settle allegations that it violated the FCA and the Financial Institutions Reform, Recovery and Enforcement Act it used data in violation of its government contracts. The government alleged that over a month-long period, the company improperly accessed, retained, and anonymized credit card data it received under various government contracts, which it subsequently used to create proxy data that was incorporated into products and services sold to commercial customers. The company failed to disclose this behavior both to the government and to the commercial clients to whom it sold the products.[41]
- On April 23, a company responsible for managing and operating a National Nuclear Security Administration site agreed to pay $18.4 million to settle liability for overpayments that resulted from production technicians submitting falsified timesheets over a six-year period. The company received credit under the settlement for self-disclosing the misconduct, cooperating with the government’s investigation, and for undertaking remediation efforts (including terminating the personnel who engaged in the misconduct).[42]
- On June 6, a Canadian manufacturer of protective head gear for U.S. military and law enforcement use agreed to pay approximately $2.5 million to resolve claims that it used foreign-sourced materials in its production of helmet inserts in violation of the Berry Amendment. The company sold its products to the U.S. military under the Defense Logistics Agency’s Special Operational Equipment Tailored Logistic Support Program, which requires that textiles be sourced from the United States in compliance with the Berry Amendment. The government initiated an investigation involving the US Department of Defense, the Defense Criminal Investigative Service, and the Department of the Army Criminal Investigation Division after receiving a complaint from the DLA hotline. The settlement amount reflected that the company accepted responsibility, cooperated with the government’s investigation, and implemented compliance measures.[43]
- On June 7, a conglomerate of three medical practice and management groups operating urgent care practices in New Jersey and New York agreed to pay over $12 million to resolve allegations that they submitted false claims for reimbursement of COVID-19 tests to a program that funds COVID-19 testing for uninsured individuals. The government alleged that operators did not adequately confirm whether test recipients had health insurance coverage before submitting their claims to the program, resulting in the erroneous submission of claims for insured persons. It also alleged that the operators caused laboratories to submit false claims for those COVID-19 tests by providing requisition forms that inaccurately indicated the test recipients were uninsured. The operators received credit in the settlement for their voluntary disclosure, cooperation, and remediation efforts. The settlement also resolves a qui tam suit brought by a patient, who will receive approximately $2 million of the settlement.[44]
- On June 17, two consulting companies agreed to settle allegations that they violated the False Claims by failing to meet cyber security requirements as part of the administration of the application system for the Emergency Rental Assistance Program. As part of the settlement, both companies admitted that they failed to satisfy their obligation to complete required cybersecurity testing for the systems. One company agreed to pay $7.6 million and the second agreed to pay $3.7 million as part of the settlement. The settlement also resolves a qui tam lawsuit brought by an entity owned by a former employee of one of the companies, which will receive a share of approximately $1.9 million of the settlement.[45]
- On June 21, two Wisconsin and Connecticut-based aerospace and parts companies agreed to pay $70 million to resolve False Claims Act allegations that they overcharged the Navy for spare parts and materials needed to repair and maintain Navy aircrafts. According to the government’s allegations, the two companies, which were both wholly-owned subsidiaries of the same parent company, knowingly entered into a contract under which one would purchase parts from the other at a markup. The purchasing company then submitted cost vouchers to the Navy for reimbursement. The settlement also resolves a qui tam suit but does not specify the relator’s share of the recovery.[46]
C. Other
- On January 31, an automobile accessory company agreed to pay $3 million to resolve allegations that it knowingly failed to pay antidumping and countervailing duties on materials it imported from China. In particular, the government alleged that the company failed to take any action after being informed that it was not paying the appropriate duties on extruded aluminum components from January 2012 through July 2021. This resolved a qui tam action brought by a former employee who will receive $510,000 plus $75,000 in legal fees as part of the settlement.[47]
- On February 29, two individuals in Colorado agreed to pay $3.5 million to resolve allegations that they defrauded the federal government by tampering with rain gauges. The government alleged that the two individuals were part of a conspiracy to tamper with the rain gauges by various means in order to make it appear as though there was below-average rainfall. Doing so would allow them to take advantage of a federal program that pays indemnities to farmers when there is below-average precipitation. In addition to civil penalties, the two individuals pled guilty to criminal charges for which they received prison sentences and were ordered to pay an additional $3.1 million in restitution. The settlement also resolves a qui tam suit for which the relator’s estate will receive approximately $500,000.[48]
- On March 13, a construction company agreed to pay $2.5 million plus interest to resolve allegations that it violated FCA by taking out EIDL and PPP loans that it was not entitled to. Specifically, the government alleged that the company’s owner falsely certified in loan applications that he had not been convicted of a felony involving fraud within the last five years even though he had pled guilty and was convicted of a fraud-related felony charge less than three years before the first loan application. This settlement also resolves a qui tam suit for which the relator will receive approximately $250,000.[49]
- On March 21, a New Jersey chemical importer and its owner agreed to pay $3.1 million to resolve claims that it fraudulently underpaid customs duties. In particular, the government alleged that the importer conspired with a Chinese vendor to mislabel imported chemicals, including hazardous chemicals, and submitted falsified documents to customs brokers. This settlement also resolves a qui tam suit for which the relator will receive $600,000. The company’s owner additionally pled guilty to wire fraud.[50]
- On May 2, a German airline and its Minneapolis-based subsidiary agreed to pay $26.8 million to resolve allegations that it failed to remit to the federal government mandatory travel fees that the airline collected from passengers. In particular, the government alleged that from 2012 to 2018, the company collected fees such as those owed to U.S. Customs and TSA but did not pay those fees to the appropriate government entities. The settlement resolved a qui tam lawsuit for which the relator will receive approximately $4.8 million.[51]
- On May 7, a now-bankrupt lender agreed to pay up to $120 million over two settlements to resolve allegations that it submitted false claims for loan forgiveness, loan guarantees, and processing fees to the government under PPP. Under the first settlement, the company agreed to pay up to $63.2 million to resolve allegations that the company inflated PPP loans, causing the Small Business Administration to guarantee and forgive greater loan amounts than borrowers were entitled to receive. And, under the second settlement, the company agreed to pay up to $56.7 million to resolve allegations that the company knowingly failed to implement adequate fraud controls to comply with its regulatory obligations to prevent fraudulent borrowers from seeking PPP benefits. Because the settlement gives the government an unsecured bankruptcy claim, the ultimate settlement amount will depend on the lender’s overall assets. The settlement resolves two qui tam actions for which the relators will receive a portion of the proceeds.[52]
- On June 12, multiple nonprofit organizations, including two private country clubs and two homeowners associations, paid approximately $5.8 million to settle allegations that they violated the False Claims Act by knowingly submitting false claims and obtaining PPP loans for which they were not eligible. The settlements also resolved a qui tam action for which the relator will receive approximately $700,000 of the total recovery.[53]
- On June 20, four restaurants, two fur apparel distributors, and five individuals agreed to pay approximately $4.6 million to settle allegations that they inflated payroll figures in their PPP loan and forgiveness applications. According to the government, the defendants misrepresented that family members and acquaintances were employed by the businesses, listed the same individuals as “full-time employees” of multiple businesses, inflated payroll figures, and improperly sought loan forgiveness for payroll costs that exceeded the maximum allowed. The settlement also resolved a qui tam lawsuit brought by a former manager at two of the restaurants, but does not specify what, if any, portion of the recovery he will receive.[54]
III. Cyber-Fraud Initiative Updates
The first half of 2024 witnessed notable developments in DOJ’s Civil Cyber-Fraud Initiative, an effort we reported on in our 2023 Year-End Update. The Initiative, launched on October 6, 2021, aims to use the FCA to pursue cybersecurity-related fraud by government contractors and grant recipients that are “knowingly providing deficient cybersecurity products or services, knowingly misrepresenting their cybersecurity practices or protocols, or knowingly violating obligations to monitor and report cybersecurity incidents and breaches.”[55] In February 2024, Principal Deputy Assistant Attorney General Brian Boynton emphasized that DOJ “will continue to dedicate resources to investigating companies that fail to comply with their cybersecurity obligations.”[56]
A. DOJ Intervenes in First-Of-Its-Kind Cybersecurity Suit Since Launch of Civil Cyber-Fraud Initiative
In the same month in which DOJ re-emphasized its commitment to cybersecurity enforcement, DOJ intervened in a first-of-its-kind qui tam lawsuit, alleging that the Georgia Institute of Technology and Georgia Tech Research Corporation failed to comply with mandatory cybersecurity controls in their Department of Defense (DOD) contracts. In United States ex rel. Craig v. Georgia Tech Research Corporation, et al., the Associate Director of Cybersecurity at Georgia Tech and Principal Information Security Engineer brought the suit in July 2022 against research organizations for allegedly failing to secure and interact with government information and data under standards by the National Institute of Standards and Technology (NIST).
DOD contractors must comply with DFARS 252.204-7012 (“Safeguarding Covered Defense Information and Cyber Incident Reporting”), which requires contractors provide “adequate security” to safeguard the defense information they handle during the course of their work for the DOD. In turn, “adequate security” is defined, at a minimum, as implementation of NIST Special Publication 800-171 (NIST SP 800-171), which has 110 security requirements relating to, among other things, identification and authentication measures; audit and accountability; and system and communications protection measures. The lawsuit alleges that defendants’ internal assessors assigned to determine compliance with NIST were not qualified, and they were pressured interpret the NIST controls to justify certain actions taken in labs as compliant.
The government’s deadline to serve defendants with a complaint-in-intervention is August 22, 2024.
B. Potential Civil Cyber-Fraud Initiative Case on Stay
Similarly, in our 2023 Year-End Update we also reported on an unsealed qui tam complaint against Penn State by a relator who alleged that the university submitted false cybersecurity certifications to DOD. Following a 90-day stay to allow the government additional time to determine whether it will intervene, the parties’ joint written status report updating the court on any developments is due by August 5, 2024.[57]
IV. Legislative and Policy Developments
A. Federal Policy and Legislative Developments
1. Proposed Revisions to Medicare Overpayment Rules
On July 10, 2024, the Centers for Medicare and Medicaid Services (“CMS”) issued a proposed rule regarding the Physician Fee Schedule (“PFS”), which governs Medicare payments for the services of physicians and other healthcare professionals.[58] While changes to the PFS were the headline purpose of the proposed rule, the rule also would bring about significant changes to existing provisions governing healthcare providers’ return of overpayments under Medicare Parts A and B. By way of context, the Affordable Care Act (“ACA”) requires providers to return overpayments to the government within 60 days of the date on which the overpayments are “identified,” and specifies that an overpayment not returned by the appropriate deadline counts as an “obligation” for purposes of the reverse FCA, which prohibits knowing and improper avoidance of an obligation to pay money to the government.[59]
The ACA does not specify what it means to “identify” an overpayment.[60] As originally promulgated, regulations governing the return of overpayments by the Medicare program stated that a provider “identifies” an overpayment when it “has determined, or should have determined through the exercise of reasonable diligence, that [it] has received an overpayment.”[61] In a proposed rule issued in late 2022, CMS proposed to replace this looser standard of knowledge with the relatively more stringent definition of “knowing” and “knowingly” contained in the FCA.[62] This change came in direct response to a district court decision that struck down the “reasonable diligence” standard as permitting the government to premise FCA liability on nothing more than negligence, when the FCA requires a minimum of reckless disregard.[63] That decision and CMS’s response to it, however, left unaddressed a core problem confronting large organizations that face overpayment risks—namely, that it can take much longer than 60 days to determine whether an overpayment has occurred, and the running of that clock without any action to return monies to the government is very often a sign that a good-faith investigation into potential overpayments remains underway, not that overpayments were quickly identified and are being concealed. CMS had previously acknowledged that internal investigations into potential overpayments could take around 180 days, but there was neither a requirement that such investigations be completed in that timeframe nor an explicit provision tolling the deadline for return of overpayments pending such investigations.[64]
The new proposed rule would permit the suspension of the 60-day clock to allow companies to conduct internal investigations, but the devil remains in the details. In particular, in order for the deadline to be suspended, a company would have to have already identified at least one overpayment and be in the midst of a “good-faith investigation to determine the existence of related overpayments,” and would have to actually conduct such a good-faith investigation.[65] And the deadline for returning overpayments would only be suspended until, at the latest, 180 days after the date on which the company identified the initial overpayment that triggered the broader investigation.[66] While these changes enhance incentives for companies to conduct investigations into potential overpayments by extending the reporting deadline pending the completion of such investigations, the reality is that even 180 days may prove an insufficient amount of time for such investigations to fully run their course in large companies. The 180‑day cutoff risks being weaponized by qui tam relators claiming that any investigation that takes longer than 180 days must not have been conducted in “good faith” under the new rule, and that thus any overpayments not returned after the expiration of the 180-day window should form the basis for reverse FCA liability.
CMS is accepting comments on the proposed rule until September 9, 2024.
2. DOJ Whistleblower Reward Program and Voluntary Self-Disclosures Pilot Program for Individuals
Qui tam cases account for the majority of FCA cases initiated in any given year, as well as for the bulk of the monies the government recovers from FCA matters through settlement or judgment. In 2023, qui tam cases represented about 59% of the new FCA cases filed, and about 87% of the recoveries obtained. The FCA qui tam framework has no counterpart in U.S. criminal statutes, but DOJ recently has taken steps to develop a more formal policy for whistleblower awards in the criminal context. In March 2024, DOJ announced the creation of a pilot program that would reward a whistleblower with a portion of the resulting forfeiture if he or she helps DOJ discover significant corporate or financial misconduct.[67] In announcing this program, DOJ noted the successes of similar programs created at the SEC, CFTC, IRS, and FinCEN but acknowledged that those programs were limited to misconduct within those agencies’ jurisdictions. DOJ also noted that qui tam whistleblower initiatives are limited to those actions where fraud against the government is alleged. Thus, DOJ’s new initiative would “fill[] these gaps” to “address the full range of corporate and financial misconduct that the Department prosecutes.”[68]
While details on this pilot program are still forthcoming, the announcement identified important “guardrails.”[69] Payments would be made (1) only after all victims have been properly compensated; (2) only to those who submit truthful information not already known to the government; (3) only to those not involved in the criminal activity itself; and (4) only in cases where there is not an existing financial disclosure incentive—including qui tam awards or an award under another federal whistleblower program.[70] Deputy Attorney General Monaco also told potential future whistleblowers that DOJ was especially interested in information regarding “[c]riminal abuses of the U.S. financial system; [f]oreign corruption cases outside the jurisdiction of the SEC, including FCPA violations by non-issuers and violations of the recently enacted Foreign Extortion Prevention Act; and [d]omestic corruption cases, especially involving illegal corporate payments to government officials.”[71]
Relatedly, in April 2024, DOJ’s Criminal Division announced a pilot program that would extend the benefits of voluntary self-disclosure to individuals who (1) voluntarily, (2) truthfully, and (3) completely self-disclose original information regarding misconduct that was unknown to the department in certain high-priority enforcement areas, (4) fully cooperate and are able to provide substantial assistance against those equally or more culpable, and (5) forfeit any ill-gotten gains and compensate victims.[72] To qualify, a disclosure must relate to at least one of six areas of DOJ focus:
- Schemes involving financial institutions (g., money laundering, criminal compliance-related schemes);
- Schemes relating to the integrity of financial markets involving financial institutions, investment advisors or funds, or public or large private companies;
- Foreign corruption schemes (e.g., violations of the Foreign Corrupt Practices Act, Foreign Extortion Prevention Act, and associated money laundering);
- Health care fraud and kickback schemes;
- Federal contract fraud schemes; or
- Domestic corruption schemes involving bribes or kickbacks paid by or through public or private companies.
Deputy Attorney General Lisa Monaco noted that at least two U.S. Attorney’s offices—in the Southern District of New York and the Northern District of California—established similar programs earlier in the year.[73]
Beyond their significance for DOJ’s criminal enforcement efforts, these developments have important implications for FCA practice as well. Because the FCA penalizes fraud, the conduct at issue in an FCA investigation can sometimes be of interest to criminal authorities too. Yet the risks for a would-be whistleblower in coming forward are magnified when the alleged conduct carries potential criminal, in addition to civil, liability. In such a scenario, the possibility that DOJ will decide the relator has unclean hands carries not just the potential for criminal liability, but also the prospect of outright denial of a qui tam award. The FCA explicitly provides that a relator who is “convicted of criminal conduct arising from his or her role in the [FCA] violation . . . shall be dismissed from the civil action and shall not receive any share of the proceeds of the action.”[74] The new criminal whistleblower pilot program creates an additional financial incentive for reporting misconduct that operates independently of the qui tam mechanism. Alongside that pilot program, the individual voluntary self-disclosure pilot program stands to remove the disincentive that otherwise exists in the form of qui tam award denial in the event of a criminal conviction. Relators may prove more forthcoming about alleged conduct and their own roles in it, if both non-prosecution and financial gain remain on the table. And the carve-out in the pilot whistleblower program for individuals already covered by another whistleblower regime will likely do little to stop relators from making simultaneous reports to both civil and criminal authorities in the hope of maximizing their chances of a recovery.
B. State Legislative Developments
There were no major developments with respect to state FCA legislation in the second half of 2022. HHS-OIG provides an incentive for states to enact false claims statutes in keeping with the federal FCA. If HHS OIG approves a state’s FCA, the state receives an increase of 10 percentage points in its share of any recoveries in cases involving Medicaid. The lists of “approved” state false claims statutes increased to 23 with the approval of Connecticut’s statute this year; while six states remain on the “not approved” list.[75] The other 21 states have either not enacted a state analogue or have not submitted the statue for approval.
V. Case Law Developments
A. U.S. Supreme Court Grants Certiorari in E-Rate Fraud Claims Case
In June, the Supreme Court granted a petition for a writ of certiorari filed by Wisconsin Bell on the question whether reimbursement requests submitted to the Federal Communications Commission’s E-rate program are “claims” under the FCA. See United States ex rel. Heath v. Wis. Bell, 92 F.4th 654, 657 (7th Cir. 2024), cert. granted, 2024 WL 3014477 (U.S. June 17, 2024). The $4.5 billion E-rate program, established under the Telecommunications Act of 1996, provides discounted services to eligible schools and libraries for which service providers competitively bid on pricing and subsidize cost of service. It is funded by private money and administered by a non-profit company. (Note: Gibson Dunn represents Wisconsin Bell in this matter.)
After relator Todd Heath alleged in 2008 that Wisconsin Bell violated the FCA by over-charging schools and libraries, causing the federal government to pay more than it should have, id. at 658, Wisconsin Bell argued that the relator could not satisfy the FCA because, among other things, the E-rate program does not involve government funds, and reimbursement requests are not “claims” within the meaning of the FCA. The district court granted summary judgment for Wisconsin Bell, holding that the relator had not established falsity, scienter, or harm to the government fisc. Id. The Seventh Circuit reversed the district court’s grant of summary judgment. Id. at 671. By reinstating the relator’s claims, the Seventh Circuit created a circuit split with the Fifth Circuit, which had previously held that the FCA does not apply to E-Rate reimbursement requests because the government lacks a financial stake in the allegedly lost funds. See generally United States ex rel. Shupe v. Cisco Sys., Inc., 759 F.3d 379, 388 (5th Cir. 2014).
The certiorari petition was granted on June 17, and oral argument is set for November 4, 2024.
B. The Seventh Circuit Remands on Causation and Upholds Damages Award Against Eighth Amendment Challenge
The Seventh Circuit heard argument in Stop Ill. Health Care Fraud, LLC v. Sayeed, 100 F.4th 899 (7th Cir. 2024) on the FCA causation issue but declined to take a position and remanded to the district court for further argument.
In Stop Ill. Health Care Fraud, Management Principles Inc. (“Management Principles”), a healthcare management company which provided home-based medical services to Medicare recipients, as well as its two subsidiaries and owner, faced AKS allegations for paying Healthcare Consortium of Illinois (“Healthcare Consortium”) $5,000 monthly in exchange for patient referrals. Id. at 902–03. The company allegedly relied on referrals from Healthcare Consortium, a healthcare diagnostic organization, that would refer seniors to local in-home healthcare providers. Id. Management Principles allegedly paid this organization $90,000 for referrals and access to client data, and allegedly billed the federal government over $700,000 for services provided to clients referred by Healthcare Consortium. Id. at 903. Following a bench trial, the district court found that this scheme violated the AKS by paying to induce referrals for medical services. Id. at 904. The district court also found the defendants liable under the FCA for submitting claims for payments stemming from an unlawful referral arrangement. Id. The district court imposed a judgment of nearly $6,000,000, comprised of the sum of per-claim penalties of $5,500 per claim and treble the value of the Medicare claims at issue. Id. The defendants appealed, challenging causation and the award of damages and penalties, “arguing that it [was] constitutionally excessive under the Eighth Amendment and improperly divorced form the actual loss incurred by the government.” Id. at 906.
The Seventh Circuit held the “resulting from” language in the AKS means “at a minimum, every claim that forms the basis of FCA liability must be false by virtue of the fact that the claims are for services that were referred in violation of the Anti-Kickback Statute.” Id. at 908. The court explained that it was “not able to determine with confidence whether any of the services represented in the plaintiff’s loss spreadsheet were provided to patients lawfully referred to the defendants by the [Healthcare] Consortium.” Id. at 909. The court remanded the case back to the district court for the limited task of determining which claims, if any, were the result of a referral process outside the kickback scheme. Id. at 909–10. Thus, in doing so, the Seventh Circuit declined to weigh in conclusively on the proper causation standard for AKS-predicated FCA claims, id. at 909, leaving the Seventh Circuit without a definitive position on either side of the deepening circuit split on this issue, which we covered in our 2023 Mid-Year and End-Year Updates. In declining to take a position, however, the Seventh Circuit signaled that, if it does take a side in the debate, it is unlikely to hold that the existence of a kickback “taints” all subsequent claims for payment, regardless of any causal connection between the kickback and the claims. The court made clear that “[t]hat broad suggestion . . . is inconsistent with [the FCA’s] directive that a false claim must ‘result[] from’ an unlawful kickback.” Id. (second alteration in original). We will continue to closely monitor developments around this issue, including as the related Regeneron case in the First Circuit proceeds to oral argument this summer.
Additionally, the Seventh Circuit also addressed whether the nearly $6 million judgment was unconstitutionally excessive under the Eight Amendment. The court held that the judgment did not violate the Eighth Amendment’s Excessive Fines Clause, but that the district court still erred by calculating those damages based on Medicare claims that might not have been related to the kickback scheme. Id. at 906–07. The court explained that while the Seventh Circuit has not explicitly held whether the Excessive Fines Clause applies to civil penalties under the FCA, the judgment in this particular case would not violate the clause even if it were to apply. Id. The Seventh Circuit held that because the defendants established an extensive scheme that defrauded the government, exploited the private health information of seniors, and undermined the public’s faith in government programs, the judgment was not “grossly disproportional to the gravity of the defendant’s offense,” thereby passing Eighth Amendment scrutiny. Id..
C. The Sixth Circuit Holds Courts Can Require Plaintiffs Take All Reasonable Steps to Dismiss an FCA Suit, Including Seek Government Consent
A relator cannot unilaterally settle FCA claims without the government’s consent. See 31 U.S.C. § 3730 (requiring the government’s consent to any voluntary dismissal of a qui tam case). In State Farm Mut. Auto. Ins. Co. v. Angelo, the Sixth Circuit clarified what steps a court can require a party take to dismiss a FCA suit. 95 F.4th 419 (6th Cir. 2024).
After State Farm sued Michael Angelo, alleging RICO violations, the parties entered into a settlement agreement. Id. at 424. In the agreement, Angelo agreed to take “all steps necessary” to release claims against State Farm. Id. Before the agreement was signed, Angelo filed an FCA suit against State Farm. Id. Because qui tam suits are required to be filed under seal, State Farm was unaware of the case until after the RICO settlement agreement was signed and the complaint was unsealed and served on State Farm. Id. In the ensuing litigation over State Farm’s motion to dismiss the FCA claims, Angelo argued that he could not dismiss the claims because the FCA prohibits relators from dismissing qui tam cases without the government’s consent. Id. at 425. The district court granted State Farm’s motion, ordering Angelo to take all steps necessary to dismiss his FCA claims, including seeking the necessary government consent. Id.
On appeal, the Sixth Circuit upheld the district court’s orders enforcing the RICO settlement agreement. The court explained that while “the FCA statute demands government consent before a qui tam relator can dismiss an FCA claim[,]” the law does not “prevent[] a relator from seeking the required consent or prohibit[] a district court from ordering a relator to seek such consent.” Id. at 429–30 (emphasis in original). The Sixth Circuit rejected Angelo’s argument that under this interpretation, the settlement agreement violates the public policy rationale behind the FCA. Id. at 430. The court held that the “primary goals of the FCA are to incentivize private individuals to bring suit and to alert the government to potential fraud,” goals which the RICO settlement did not undermine. Id. Because Angelo had filed the FCA suit two years before the settlement was signed, both Angelo and the government had ample time to investigate the claims. Id. at 431. The court further explained that even if there had not been ample time, the government still had the opportunity to deny consent to dismiss or to file its own FCA claims, as it was not a party to the RICO settlement and thus was not bound by agreement requiring Angelo to take steps to effectuate dismissal of the qui tam case. Id. at 432.
D. The Second Circuit Clarifies When a Worker Engages in “Protected Activity”
The FCA prohibits retaliation against employees who report potential FCA violations. See 31 U.S.C. § 3730(h)(1). In Pilat v. Amedisys, Inc., workers claimed they were fired in retaliation for raising concerns about certain practices of Amedisys, a home health and hospice company. No. 23-566, 2024 WL 177990 (2d Cir. Jan. 17, 2024). The workers alleged that they disclosed to superiors that Amedisys falsely certified unqualified patients for home care, provided unnecessary and improper treatment, falsified time records, and manipulated patient records. Id. at *1. These schemes allegedly resulted in fraudulent bills to the government for reimbursement under the Medicare and Medicaid programs. Id. The workers alleged that after they expressed concerns over the unethical nature of these practices and their effects on the health of patients and refused to comply with instructions to carry out these practices, Amedisys fired them. Id. at *1–2. The district court held that the Plaintiffs did not have a valid retaliation claim since they did not “engage in protected activity under the statute.” Id. at *1. The court explained that the complaints were “more appropriately characterized as concerns about patient care[,]” and “did ‘not have anything to do with potential false claims.’” Id. at *9 (citing United States v. Amedisys, No. 17-CV-136, 2023 WL 2481144, at *9 (W.D.N.Y. Mar. 13, 2023)).
The Second Circuit reversed and explained that “relators engage in protected activity if they engage in ‘efforts to stop 1 or more violations of’ the FCA.” Pilat, 2024 WL 177990 at *2 (quoting 31 U.S.C. § 3730(h)(1)). Such efforts can include raising concerns to supervisors or refusing to engage in violative practices. Id. Because in this case the workers refused to comply with instructions to engage in conduct that would have violated the FCA, they made “efforts to stop 1 or more violations,” even if their main concern was the safety of patients. Id. The court further rejected the district court reasoning that the Plaintiffs only raised concerns of patient care, not fraud. Even if the complaints were based on concerns of patient care, the Plaintiffs still raised concerns that the amounts billed to the government did not match the actual time spent treating patients, a concern which clearly implicated potential fraud. Id.
E. The Second Circuit Affirms Heightened Scienter Under the Anti-Kickback Statute
While the FCA is a civil statute, DOJ and relators often allege that violations of the AKS—a criminal statute—are what made certain claims for payment false. The two statutes contain different scienter requirements. The FCA imposes liability on any person who “knowingly presents . . . a false or fraudulent claim [to the government] for payment or approval.” 31 U.S.C. § 3729(a)(1)(A). The FCA defines “knowingly” to mean that a person (1) “has actual knowledge of the information,” (2) “acts in deliberate ignorance of the truth or falsity of the information,” or (3) “acts in reckless disregard of the truth or falsity of the information,” and “require[s] no proof of a specific intent to defraud.” 31 U.S.C. § 3729(b)(1)(A-B). The Supreme Court recently clarified that the FCA’s “knowingly” standard refers to the defendant’s knowledge and subjective beliefs, not what an objectively reasonable person might have known or believed. United States ex rel. Schutte v. SuperValu Inc., 143 S. Ct. 1391, 1404 (2023). The AKS, on the other hand, imposes liability on any person who “knowingly and willfully makes or causes to be made any false statement or representation of a material fact in any application for any benefit or payment under a Federal health care program.” 42 U.S.C. § § 1320a–7b. In United States ex rel. Hart v. McKesson Corp., the Second Circuit affirmed a key decision interpreting the willfulness requirement in cases where an FCA violation is premised on a violation of the AKS. 96 F.4th 145 (2d Cir. 2024).
Plaintiffs alleged that Defendants operated an illegal kickback scheme in violation of the AKS and the FCA. Id. at 150. According to the complaint, McKesson offered business management tools for free to customers who agreed to solely purchase drugs from McKesson. Id. at 151–52. Plaintiffs alleged that this scheme violated the AKS and thus the FCA. Id. The district court granted McKesson’s motion to dismiss, holding that to act “willfully” as required by the AKS, “a defendant must act knowing that its conduct is, in some way, unlawful,” a standard Hart failed to plead. Id. at 150. The district court held that because the FCA claim was premised on the AKS claims alone, the defendant failed to plausibly allege an FCA claim. Id.
The Second Circuit affirmed and interpreted the AKS’s “willful” requirement to mean that “a defendant must act with a ‘bad purpose’” and “‘with knowledge that his conduct was unlawful.’” Id. at 157 (quoting Bryan v. United States, 524 U.S. 184, 191 (1998)). The court held that “to violate the AKS, a defendant must act knowing that his conduct is unlawful, even if the defendant is not aware that his conduct is unlawful under the AKS specifically.” Id. at 154 (citing Pfizer v. U.S. Dep’t of Health & Hum. Servs., 42 F.4th 67, 77 (2d Cir. 2022)). The court held that “a defendant’s knowledge of his general legal obligations is not enough if he does not also know that his actions violate those obligations,” id. at 158, and affirmed the dismissal of Hart’s claim for failure to plead willfulness adequately, id. at 157–59. Notably, the Second Circuit looked to the specific knowledge of individuals other than the relator when determining whether the Plaintiff adequately pleaded willfulness. Id. at 160–62 (rejecting relator’s argument that he sufficiently alleged scienter because he pleaded that he told a supervisor that he thought certain conduct violated company policies).
VI. Conclusion
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2024 False Claims Act Year-End Update, which we will publish in early 2025.
[1] These figures, and the summaries that follow, cover the period from January 1, 2024 through June 30, 2024 and focus on settlements valued at $2 million or more.
[2] See Press Release, U.S. Atty’s Office for the Dist. of Del., ChristianaCare Pays $42.5 Million To Resolve Health Care Fraud Allegations (Jan. 4, 2024), https://www.justice.gov/usao-de/pr/christianacare-pays-425-million-resolve-health-care-fraud-allegations-0.
[3] See Press Release, U.S. Atty’s Office for the Middle Dist. of Fl., Florida Research Hospital Agrees To Pay More Than $19.5 Million To Resolve Liability Relating To Self-Disclosure Of Improper Billing For Clinical Trial Costs (Jan. 4, 2024), https://www.justice.gov/usao-mdfl/pr/florida-research-hospital-agrees-pay-more-195-million-resolve-liability-relating-self.
[4] See Press Release, U.S. Atty’s Office for the Middle Dist. of Tenn., Memphis-Based Methodist Le Bonheur Healthcare and Methodist Healthcare-Memphis Hospitals Pay $7.25 Million to Settle Allegations that They Violated the False Claims Act (Jan. 4, 2024), https://www.justice.gov/usao-mdtn/pr/memphis-based-methodist-le-bonheur-healthcare-and-methodist-healthcare-memphis.
[5] See Press Release, Dep’t of Justice, Home Healthcare Company Agrees to Pay Nearly $10 Million to Resolve False Claims Act Allegations Relating to Its Participation in the Energy Employees Occupational Illness Compensation Program (Jan. 5, 2024), https://www.justice.gov/opa/pr/home-healthcare-company-agrees-pay-nearly-10-million-resolve-false-claims-act-allegations.
[6] See Press Release, Dep’t of Justice, New Jersey Laboratory and Its Owner and CEO Agree to Pay Over $13 Million to Settle Allegations of Kickbacks and Unnecessary Testing (Jan. 10, 2024), https://www.justice.gov/opa/pr/new-jersey-laboratory-and-its-owner-and-ceo-agree-pay-over-13-million-settle-allegations.
[7] See Press Release, U.S. Atty’s Office for the Dist. of N.J., New Jersey Hospital and Investors to Pay United States $30.6 Million for Alleged False Claims (Jan. 16, 2024), https://www.justice.gov/usao-nj/pr/new-jersey-hospital-and-investors-pay-united-states-306-million-alleged-false-claims#:~:text=Alleged%20False%20Claims-,New%20Jersey%20Hospital%20and%20Investors%20to%20Pay%20United,Million%20for%20Alleged%20False%20Claims&text=NEWARK%2C%20N.J.%20%E2%80%93%20A%20New%20Jersey,violations%2C%20U.S.%20Attorney%20Philip%20R.
[8] See Press Release, U.S. Atty’s Office for the Dist. of Idaho, AmeriHealth Clinics Consent to a $2 Million Judgment to Resolve Healthcare Fraud Allegations (Jan. 17, 2024), https://www.justice.gov/usao-id/pr/amerihealth-clinics-consent-2-million-judgment-resolve-healthcare-fraud-allegations.
[9] See Press Release, U.S. Atty’s Office for the Dist. of Pa., Current and Former Owners of Center City Philadelphia Pharmacy Agree to Pay Over $4.6 Million to Resolve Civil Investigations of Improper Medicare and Medicaid Billing (Jan. 23, 2024), https://www.justice.gov/usao-edpa/pr/current-and-former-owners-center-city-philadelphia-pharmacy-agree-pay-over-46-million.
[10] See Press Release, U.S. Atty’s Office for the Dist. of S.C., Durable Medical Equipment Companies to Pay Millions in False Claims Settlement (Jan. 26, 2024), https://www.justice.gov/usao-sc/pr/durable-medical-equipment-companies-pay-millions-false-claims-settlement.
[11] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Ky., Kentucky Lab Agrees to $4.9 Million Civil Judgment and Drug Treatment Center Enters Settlement to Pay $2.2 Million to Resolve False Claims Act Allegations (Jan. 30, 2024), https://www.justice.gov/usao-edky/pr/kentucky-lab-agrees-49-million-civil-judgment-and-drug-treatment-center-enters.
[12] See Press Release, U.S. Atty’s Office for the Mid. Dist. of Pa., Penn State Health Agrees To Pay More Than Eleven Million Dollars Following Its Voluntary Disclosure Of Improper Billings Related To Medicare Annual Wellness Visit Services (Feb. 7, 2024), https://www.justice.gov/usao-mdpa/pr/penn-state-health-agrees-pay-more-eleven-million-dollars-following-its-voluntary.
[13] See Press Release, U.S. Atty’s Office for the Southern Dist. of N.Y., U.S. Attorney Announces $25.5 Million Settlement With Durable Medical Equipment Supplier Lincare Inc. For Fraudulent Billing Practices (Feb. 15, 2024), https://www.justice.gov/usao-sdny/pr/us-attorney-announces-255-million-settlement-durable-medical-equipment-supplier.
[14] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Ky., Lexington Lab Agrees to $10.4 Million in Civil Judgments to Resolve False Claims Act Allegations; Owner and Lab Officer Sentenced to Prison (Feb. 16, 2024), https://www.justice.gov/usao-edky/pr/lexington-lab-agrees-104-million-civil-judgments-resolve-false-claims-act-allegations.
[15] See Press Release, U.S. Dep’t of Justice, Opioid Manufacturer Endo Health Solutions Inc. Agrees to Global Resolution of Criminal and Civil Investigations into Sales and Marketing of Branded Opioid Drug (Feb. 29, 2024), https://www.justice.gov/opa/pr/opioid-manufacturer-endo-health-solutions-inc-agrees-global-resolution-criminal-and-civil; Settlement Agreement, Endo Health Solutions Inc., https://content.govdelivery.com/attachments/USDOJOPA/2024/02/29/file_attachments/2799079/Endo%20Civil%20FCA%20Settlement%20Agmt%20%28Fully%20Executed%29.pdf.
[16] See Press Release, U.S. Atty’s Office for the Northern Dist. of Ga., Georgia Laboratory Owner Pleads Guilty to Felony Charge and Agrees to Pay $14.3 Million to Resolve False Claims Act Allegations (Feb. 28, 2024), https://www.justice.gov/usao-ndga/pr/georgia-laboratory-owner-pleads-guilty-felony-charge-and-agrees-pay-143-million; Settlement Agreement, U.S. Dep’t of Justice and Capstone Laboratories (Feb. 28, 2024), https://www.justice.gov/opa/media/1340321/dl?inline.
[17] See Press Release, U.S. Atty’s Office for the Eastern Dist. of N.Y., New York-Presbyterian/Brooklyn Methodist Hospital Settles Health Care Fraud Claims for $17.3 Million (Mar. 12, 2024), https://www.justice.gov/usao-edny/pr/new-york-presbyterianbrooklyn-methodist-hospital-settles-health-care-fraud-claims-173.
[18] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Generic Pharmaceuticals Manufacturer Pleads Guilty, Agrees to $1.5 Million Criminal Penalty for Distributing Adulterated Drugs and $2 Million to Resolve Civil Liability under the False Claims Act (Mar. 6, 2024), https://www.justice.gov/usao-edpa/pr/generic-pharmaceuticals-manufacturer-pleads-guilty-agrees-15-million-criminal-penalty.
[19] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Philadelphia Pharmacy Criminal Pleas and Civil Resolutions Result in Multiple Criminal Convictions and Over $4 Million Recovered (Mar. 20, 2024), https://www.justice.gov/usao-edpa/pr/philadelphia-pharmacy-criminal-pleas-and-civil-resolutions-result-multiple-criminal.
[20] See Press Release, U.S. Dep’t of Justice, Gamma Healthcare and Three of Its Owners Agree to Pay $13.6 Million for Allegedly Billing Medicare for Lab Tests That Were Not Ordered or Medically Necessary (Mar. 27, 2024), https://www.justice.gov/opa/pr/gamma-healthcare-and-three-its-owners-agree-pay-136-million-allegedly-billing-medicare-lab.
[21] See Press Release, U.S. Atty’s Office for the Southern Dist. of Ohio, Cincinnati healthcare staffing company agrees to pay $9.25 million to resolve visa fraud investigations (Mar. 25, 2024), https://www.justice.gov/usao-sdoh/pr/cincinnati-healthcare-staffing-company-agrees-pay-925-million-resolve-visa-fraud.
[22] See Press Release, U.S. Atty’s Office for the Southern Dist. of N.Y., U.S. Attorney Announces $3.1 Million False Claims Act Settlement With Radiology Company And Its CEO For Fraudulent Billing Practices (Mar. 28, 2024), https://www.justice.gov/usao-sdny/pr/us-attorney-announces-31-million-false-claims-act-settlement-radiology-company-and-its; Stipulation and Order of Settlement and Dismissal (Mar. 26, 2024), https://www.justice.gov/usao-sdny/media/1345696/dl.
[23] See Press Release, U.S. Atty’s Office for the Western Dist. of Tex., Oncology Practice, Physicians, and Reference Laboratory To Pay Over $4 Million to Settle False Claims Act Allegations (Apr. 2, 2024), https://www.justice.gov/usao-wdtx/pr/oncology-practice-physicians-and-reference-laboratory-pay-over-4-million-settle-false.
[24] See Press Release, U.S. Atty’s Office for the Central Dist. of Cal., San Gabriel Valley-Based Nursing Home Chain and Executives to Pay Over $7 Million to Settle COVID-Related False Claims Allegations (Apr. 26, 2024), https://www.justice.gov/usao-cdca/pr/san-gabriel-valley-based-nursing-home-chain-and-executives-pay-over-7-million-settle; Settlement Agreement, U.S. Dep’t of Justice and ReNew (Apr. 26, 2024), https://www.justice.gov/opa/media/1349866/dl?inline.
[25] See Press Release, U.S. Dep’t of Justice, Office of Public Affairs, Staffing Company to Pay $2.7M for Alleged Failure to Provide Adequate Cybersecurity for COVID-19 Contact Tracing Data (May 1, 2024), https://www.justice.gov/opa/pr/staffing-company-pay-27m-alleged-failure-provide-adequate-cybersecurity-covid-19-contact; Settlement Agreement, U.S. Dep’t of Justice and Insight Global (May 15, 2024), https://www.justice.gov/opa/media/1350311/dl?inline.
[26] See Press Release, U.S. Dep’t of Justice, Elara Caring Agrees to Pay $4.2 Million to Settle False Claims Act Allegations That It Billed Medicare for Ineligible Hospice Patients (May 1, 2024), https://www.justice.gov/opa/pr/elara-caring-agrees-pay-42-million-settle-false-claims-act-allegations-it-billed-medicare.
[27] See Press Release, U.S. Atty’s Office for the Dist. of N.J., Florida Businessman Daniel Hurt to Pay Over $27 Million for Medicare Fraud in Connection With Cancer Genomic Tests (May 24, 2024), https://www.justice.gov/usao-nj/pr/florida-businessman-daniel-hurt-pay-over-27-million-medicare-fraud-connection-cancer.
[28] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Mich., Local Physician and Practice Agree to Pay Over $2 Million to Settle False Claims Act Allegations (May 8, 2024), https://www.justice.gov/usao-edmi/pr/local-physician-and-practice-agree-pay-over-2-million-settle-false-claims-act/.
[29] See Press Release, U.S. Atty’s Office for the Dist. of Mass., Cape Cod Hospital to Pay $24.3 Million to Resolve Allegations That It Failed to Comply With Medicare Cardiac Procedure Rules (May 16, 2024), https://www.justice.gov/usao-ma/pr/cape-cod-hospital-pay-243-million-resolve-allegations-it-failed-comply-medicare-cardiac; Settlement Agreement, U.S. Dep’t of Justice and Cape Cod Hospital (May 16, 2024), https://www.justice.gov/usao-ma/media/1352226/dl.
[30] See Press Release, U.S. Atty’s Office for the Northern Dist. of Ohio, Cleveland Clinic to Pay Over $7 Million to Settle Allegations of Undisclosed Foreign Sources of Funding on NIH Grant Applications and Reports (May 17, 2024), https://www.justice.gov/usao-ndoh/pr/cleveland-clinic-pay-over-7-million-settle-allegations-undisclosed-foreign-sources.
[31] See Press Release, U.S. Atty’s Office for the Southern Dist. of N.Y., U.S. Attorney Announces $10.1 Million Settlement With Managed Long-Term Care Plan For Improper Receipt Of Medicaid Payments (May 23, 2024), https://www.justice.gov/usao-sdny/pr/us-attorney-announces-101-million-settlement-managed-long-term-care-plan-improper.
[32] See Press Release, Dept. of Justice, Office of Public Affairs, Medical Device Manufacturer Innovasis Inc. and Two Top Executives Agree to Pay $12M to Settle Allegations of Improper Payments to Physicians (May 29, 2024), https://www.justice.gov/opa/pr/medical-device-manufacturer-innovasis-inc-and-two-top-executives-agree-pay-12m-settle.
[33] See Press Release, U.S. Atty’s Office for the Middle Dist. of Fl., Chronic Disease Management Provider to Pay $14.9M to Resolve Alleged False Claims (June 5, 2024), https://www.justice.gov/usao-mdfl/pr/chronic-disease-management-provider-pay-149m-resolve-alleged-false-claims; Settlement Agreement, U.S. Dep’t of Justice and Bluestone National, LLC (June 5, 2024), https://www.justice.gov/opa/media/1354511/dl?inline=&utm_medium=email&utm_source=govdelivery.
[34] See Press Release, U.S. Atty’s Office for the Eastern Dist. of N.Y., Queens and Brooklyn-Based Eye Doctor Settles Health Care Fraud Claims for More Than $2.4 Million (June 6, 2024), https://www.justice.gov/usao-edny/pr/queens-and-brooklyn-based-eye-doctor-settles-health-care-fraud-claims-more-24-million.
[35] See Press Release, U.S. Atty’s Office for the Northern Dist. of Ill., Chicago Health Care Company and Its Former Owners To Pay Nearly $2 Million To Settle False Claims Act Lawsuit (June 18, 2024), https://www.justice.gov/usao-ndil/pr/chicago-health-care-company-and-its-former-owners-pay-nearly-2-million-settle-false; Settlement Agreement, U.S. Dep’t of Justice and KFM Holdings et al. (June 17, 2024), https://www.justice.gov/usao-ndil/media/1356316/dl?inline.
[36] See Press Release, U.S. Atty’s Office for the Southern Dist. Of Tex., Texas Medical Center Institutions Agree to Pay $15M Record Settlement Involving Concurrent Billing Claims for Critical Surgeries, https://www.justice.gov/usao-sdtx/pr/texas-medical-center-institutions-agree-pay-15m-record-settlement-involving-concurrent.
[37] See Press Release, U.S. Atty’s Office for the Southern Dist. of Tex., Hilcorp San Juan resolves False Claims Act claims for oil and natural gas royalty underpayments to the United States (Jan. 19, 2024), https://www.justice.gov/usao-sdtx/pr/hilcorp-san-juan-resolves-false-claims-act-claims-oil-and-natural-gas-royalty.
[38] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Mich., Federal Subcontractor Agrees to Pay $5 Million to Settle False Claims Act Allegations (Jan. 30, 2024), https://www.justice.gov/usao-edmi/pr/federal-subcontractor-agrees-pay-5-million-settle-false-claims-act-allegations.
[39] See Complaint, United States ex rel. The Arora Group, Inc. v. Planned Systems International, Inc., No. 1:21-cv-657 (May 28, 2021 E.D. Va.).
[40] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Va., Government Contractors Agree to Pay $3.9 Million to Resolve Claims of Misrepresenting Women-Owned Small Business Status (Jan. 30, 2024), https://www.justice.gov/usao-edva/pr/government-contractors-agree-pay-39-million-resolve-claims-misrepresenting-women-owned.
[41] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Va., Argus Information & Advisory Services agrees to pay $37M to settle allegations that it misused data obtained under government contracts (Mar. 12, 2024), https://www.justice.gov/usao-edva/pr/argus-information-advisory-services-agrees-pay-37m-settle-allegations-it-misused-data.
[42] See Press Release, U.S. Dep’t of Justice, Consolidated Nuclear Security Agrees to Pay $18.4 Million to Settle False Claims Act Allegations of Timecard Fraud (Apr. 23, 2024), https://www.justice.gov/opa/pr/consolidated-nuclear-security-agrees-pay-184-million-settle-false-claims-act-allegations; Settlement Agreement, Consolidated Nuclear Security, LLC (Apr. 22, 2024), https://www.justice.gov/opa/media/1349116/dl?inline.
[43] See Press Release, U.S. Atty’s Office for the Dist. of Vt. Galvion To Pay $2,495,000 To Resolve False Claims Act Allegations (June 6, 2024), https://www.justice.gov/usao-vt/pr/galvion-pay-2495000-resolve-false-claims-act-allegations.
[44] See Press Release, Dep’t of Justice, CityMD Agrees to Pay Over $12M for Alleged False Claims to the COVID-19 Uninsured Program (June 7, 2024), https://www.justice.gov/opa/pr/citymd-agrees-pay-over-12-million-alleged-false-claims-covid-19-uninsured-program.
[45] See Press Release, U.S. Atty’s Office for the Northern Dist. of N.Y., Consulting Companies to Pay $11.3 Million for Failing to Comply with Cybersecurity Requirements in Federally Funded Contract (June 17, 2024), https://www.justice.gov/usao-ndny/pr/consulting-companies-pay-113-million-failing-comply-cybersecurity-requirements.
[46] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Wis., Sikorsky Support Services, Inc. and Derco Aerospace, Inc. Agree to Pay $70 Million to Settle False Claims Act Allegations of Improper Markups on Spare Parts for Navy Trainer Aircraft (June 21, 2024), https://www.justice.gov/usao-edwi/pr/sikorsky-support-services-inc-and-derco-aerospace-inc-agree-pay-70-million-settle.
[47] See Press Release, U.S. Atty’s Office for the Western Dist. of Wash., Automobile accessory company Yakima Products Inc. settles allegations failed to pay duties on extruded aluminum from China (Jan. 31, 2024), https://www.justice.gov/usao-wdwa/pr/automobile-accessory-company-yakima-products-inc-settles-allegations-failed-pay-duties.
[48] See Press Release, U.S. Atty’s Office for the Dist. of Colo., Two Southeastern Colorado Farmers Sentenced to Federal Prison and Will Pay Over $6.5 Million for Defrauding Federal Crop Insurance Programs (Feb. 29, 2024), https://www.justice.gov/usao-co/pr/two-southeastern-colorado-farmers-sentenced-federal-prison-and-will-pay-over-65-million.
[49] See Press Release, U.S. Atty’s Office for the Dist. Of N.J., South Carolina Construction Company and Its Owner Settle Matter Alleging Receipt of Improper CARES Act Loans (Apr. 26, 2024), https://www.justice.gov/usao-nj/pr/south-carolina-construction-company-and-its-owner-settle-matter-alleging-receipt.
[50] See Press Release, U.S. Atty’s Office for the Dist. Of N.J., Owner of New Jersey Company Admits to Evading U.S. Customs Duties and His Company Agrees to $3.1 Million Settlement Agreement (Mar. 21, 2024), https://www.justice.gov/usao-nj/pr/owner-new-jersey-company-admits-evading-us-customs-duties-and-his-company-agrees-31; Information, U.S. v. George Volpe, available at https://www.justice.gov/usao-nj/media/1344671/dl?inline.
[51] See Press Release, U.S. Atty’s Office for the Dist. of D.C., Hahn Air Lines Agrees to Pay $26.8 Million to Resolve False Claims Act Liability for Its Alleged Failure to Pay Travel Fees Collected from Passengers (May 2, 2024), https://www.justice.gov/usao-dc/pr/hahn-air-lines-agrees-pay-268-million-resolve-false-claims-act-liability-its-alleged.
[52] See Press Release, U.S. Atty’s Office for the Dist. of Mass., Kabbage Agrees to Pay up to $120 Million to Resolve Allegations that it Defrauded the Paycheck Protection Program (May 13, 2024), https://www.justice.gov/usao-ma/pr/kabbage-agrees-pay-120-million-resolve-allegations-it-defrauded-paycheck-protection; Settlement Agreement, U.S. Dep’t of Justice and Kabbage, Inc. (May 13, 2024), https://www.justice.gov/usao-ma/media/1351711/dl; Settlement Agreement, U.S. Dep’t of Justice and Kabbage, Inc. (May 13, 2024), https://www.justice.gov/usao-ma/media/1351716/dl.
[53] See Press Release, U.S. Atty’s Office for the Southern Dist. of Cal., Nonprofit Organizations Pay Over $5.8 Million to Resolve Allegations of Fraudulently Obtaining Pandemic-Related Loans (June 12, 2024), https://www.justice.gov/usao-sdca/pr/nonprofit-organizations-pay-over-58-million-resolve-allegations-fraudulently-obtaining.
[54] See Press Release, U.S. Atty’s Office for the Southern Dist. of N.Y., U.S. Attorney Announces $4.6 Million False Claims Act Settlement With Restaurants, Fur Apparel Companies, And Their Owners And Managers For Submitting False Information To Obtain Paycheck Protection Program Loans (June 20, 2024), https://www.justice.gov/usao-sdny/pr/us-attorney-announces-46-million-false-claims-act-settlement-restaurants-fur-apparel.
[55] See Press Release, U.S. Dep’t of Justice, Deputy Attorney General Lisa O. Monaco Announces New Civil Cyber-Fraud Initiative (Oct. 6, 2021), https://www.justice.gov/opa/pr/deputy-attorney-general-lisa-o-monaco-announces-new-civil-cyber-fraud-initiative.
[56] See Speech, U.S. Dep’t of Justice, Principal Deputy Assistant Attorney General Brian M. Boynton Delivers Remarks at the 2024 Federal Bar Association’s Qui Tam Conference (Feb. 22, 2024), https://www.justice.gov/opa/speech/principal-deputy-assistant-attorney-general-brian-m-boynton-delivers-remarks-2024.
[57] See United States ex rel. Matthew Decker v. Pennsylvania State University, 22-cv-03895-PD (E.D. Pa. Oct. 5, 2022).
[58] See Centers for Medicare & Medicaid Servs., Calendar Year (CY) 2025 Medicare Physician Fee Schedule Proposed Rule (July 10, 2024), https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule.
[59] See 42 U.S.C. § 1320a-7k(d); 31 U.S.C. § 3729(a)(1)(G).
[60] See 42 U.S.C. § 1320a-7k(d).
[61] See, e.g., 42 C.F.R. § 422.326(c) (Medicare Advantage rule); see also 42 C.F.R. § 401.305(a)(2) (Part A and B rule), 42 C.F.R. § 423.360(c) (Part D rule) (both similar).
[62] See Dep’t of Health & Hum. Servs., Centers for Medicare & Medicaid Servs., Proposed Rule RIN 0938-AV33, at 1169 (hereinafter “Proposed PFS Rule”).
[63] See id. at 1171–72; see also UnitedHealthcare Ins. Co. v. Azar, 330 F. Supp. 3d 173, 191 (D.D.C. 2018), rev’d in part on other grounds sub nom. UnitedHealthcare Ins. Co. v. Becerra, 16 F.4th 867 (D.C. Cir. 2021).
[64] See Medicare Program; Contract Year 2015 Policy and Technical Changes to the Medicare
Advantage and the Medicare Prescription Drug Benefit Programs, 79 Fed. Reg. 29,844, 29,923
(May 23, 2014).
[65] Proposed PFS Rule at 1173.
[66] Id.
[67] See Speech, U.S. Dep’t of Justice, Deputy Attorney General Lisa Monaco Delivers Keynote Remarks at the American Bar Association’s 39th National Institute on White Collar Crime (Mar. 7, 2024), https://www.justice.gov/opa/speech/deputy-attorney-general-lisa-monaco-delivers-keynote-remarks-american-bar-associations.
[68] Id.
[69] Id.
[70] See id.
[71] Id.
[72] See Blog Post, U.S. Dep’t of Justice, Criminal Division’s Voluntary Self-Disclosures Pilot Program for Individuals (Apr. 22, 2024), https://www.justice.gov/opa/blog/criminal-divisions-voluntary-self-disclosures-pilot-program-individuals; U.S. Dep’t of Justice, Criminal Division Pilot Program On Voluntary Self-Disclosures For Individuals, https://www.justice.gov/criminal/criminal-division-pilot-program-voluntary-self-disclosures-individuals; U.S. Dep’t of Justice, Voluntary Self Disclosures for Individuals Policy (April 15, 2024), https://www.justice.gov/criminal/media/1347991/dl?inline.
[73] See Speech, U.S. Dep’t of Justice, Deputy Attorney General Lisa Monaco Delivers Keynote Remarks at the American Bar Association’s 39th National Institute on White Collar Crime (Mar. 7, 2024), https://www.justice.gov/opa/speech/deputy-attorney-general-lisa-monaco-delivers-keynote-remarks-american-bar-associations
[74] 31 U.S.C. § 3730(d)(3).
[75] State False Claims Act Reviews, HHS-OIG, https://oig.hhs.gov/fraud/state-false-claims-act-reviews/ (last visited July 1, 2024) (FCA Reviews); 42 U.S.C. § 1396h(a).
The following Gibson Dunn lawyers prepared this update: Jonathan Phillips, Winston Chan, John Partridge, James Zelenay, Michael Dziuban, Chumma Tum, Alyse Ullery, José Madrid, Mary Aline Fertin, Hayley Lawrence, Azad Niroomand, Nicole Waddick, Erin Wall, and Sara Zamani.
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues and are available to assist in addressing any questions you may have regarding these issues. Please contact the Gibson Dunn lawyer with whom you usually work, the authors, or any leader or member of the firm’s False Claims Act/Qui Tam Defense practice group:
Washington, D.C.
Jonathan M. Phillips – Co-Chair (+1 202.887.3546, jphillips@gibsondunn.com)
Stuart F. Delery (+1 202.955.8515,sdelery@gibsondunn.com)
F. Joseph Warin (+1 202.887.3609, fwarin@gibsondunn.com)
Gustav W. Eyler (+1 202.955.8610, geyler@gibsondunn.com)
Lindsay M. Paulin (+1 202.887.3701, lpaulin@gibsondunn.com)
Geoffrey M. Sigler (+1 202.887.3752, gsigler@gibsondunn.com)
Joseph D. West (+1 202.955.8658, jwest@gibsondunn.com)
San Francisco
Winston Y. Chan – Co-Chair (+1 415.393.8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415.393.8391, cstevens@gibsondunn.com)
New York
Reed Brodsky (+1 212.351.5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212.351.3850, mdenerstein@gibsondunn.com)
Alexander H. Southwell (+1 212.351.3981, asouthwell@gibsondunn.com)
Denver
John D.W. Partridge (+1 303.298.5931, jpartridge@gibsondunn.com)
Ryan T. Bergsieker (+1 303.298.5774, rbergsieker@gibsondunn.com)
Robert C. Blume (+1 303.298.5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303.298.5784, mloseman@gibsondunn.com)
Dallas
Andrew LeGrand (+1 214.698.3405, alegrand@gibsondunn.com)
Los Angeles
James L. Zelenay Jr. (+1 213.229.7449, jzelenay@gibsondunn.com)
Nicola T. Hanna (+1 213.229.7269, nhanna@gibsondunn.com)
Jeremy S. Smith (+1 213.229.7973, jssmith@gibsondunn.com)
Deborah L. Stein (+1 213.229.7164, dstein@gibsondunn.com)
Dhananjay S. Manthripragada (+1 213.229.7366, dmanthripragada@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650.849.5395, bwagner@gibsondunn.com)
*Sara Zamani is an associate in the firm’s Denver office currently admitted to practice only in California.
Who’s Who Legal and Global Investigations Review recognized 17 Gibson Dunn partners in their 2024 Investigations guide. Kelly Austin, Robert Blume, Reed Brodsky, Stephanie Brooker, Winston Chan, Michael Diamant, Nicola Hanna, Sacha Harber-Kelly, Mark Schonfeld, Benno Schwarz, Alexander Southwell, Charles Stevens, Patrick Stokes, F. Joseph Warin, Debra Wong Yang, and Finn Zeidler were recommended. Additionally, John Chesley was named a Future Leader. The guide was published April 30, 2024.
The White Collar Defense and Investigations Practice Group defends businesses, senior executives, public officials and other individuals in a wide range of investigations and prosecutions. The group includes numerous former U.S. federal and state prosecutors and officials, many of whom served at high levels within the U.S. Department of Justice, the Securities and Exchange Commission and other key investigative and prosecutorial arms of the government. Our lawyers use firsthand knowledge of how government agencies conduct investigations and prosecutions to assist our clients in navigating those processes successfully.
2023 proved that there is never a dull moment when it comes to the False Claims Act (FCA). It was an especially significant year in terms of enforcement developments.
The Department of Justice (DOJ) recovered approximately $2.7 billion through FCA settlements and judgments, making FY 2023 the 15th straight year in which recoveries exceeded $2 billion. The government and whistleblowers initiated more than 1,200 new FCA matters—a new record and a 26% increase over the previous one. Moreover, DOJ initiated 500 of these matters, the most by far in any given year since DOJ began releasing data tracking this metric. Even relator-initiated matters were significantly higher than in past years; at 712, FY 2023’s total is the third-highest since 2000. Simply put, DOJ and the relators’ bar were more active in FY 2023 than ever before. Their efforts, as in past years, were focused primarily in the healthcare space—although this past year saw a marked increase in recoveries from defense contractors, as well.
Last year was notable in other ways, too. Four years after announcing its FCA cooperation credit policy, DOJ began explicitly acknowledging certain companies’ cooperation in settlement agreements. Yet it did so with varying degrees of specificity regarding what the companies did to earn such credit, resulting in—at best—limited guidance for companies to follow in evaluating options for self-disclosure, cooperation, and remediation. Elsewhere, DOJ deepened its commitment to pursuing FCA allegations in the cybersecurity realm, and multiple states expanded their false claims laws. And while few caselaw developments could rival the two Supreme Court FCA decisions handed down in the first half of 2023, the latter half of the year still saw significant Circuit‑level decisions related to materiality, damages calculations, and the FCA’s anti‑retaliation provision, among other topics.
We cover all of this, and more, below. We begin by summarizing recent enforcement activity, then provide an overview of notable legislative and policy developments at the federal and state levels, and finally analyze significant court decisions since the publication of our 2023 Mid-Year Update.
As always, Gibson Dunn’s recent publications regarding the FCA may be found on our website, including in-depth discussions of the FCA’s framework and operation, industry-specific presentations, and practical guidance to help companies avoid or limit liability under the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
I. FCA ENFORCEMENT ACTIVITY
A. NEW FCA ACTIVITY
By a wide margin, 2023 was a record year for new FCA enforcement actions. More FCA cases were opened in 2023 than any year for which data is available. The government and qui tam relators filed 1,212 new cases, a stunning 249 more cases from the record set in 2022 (representing a 26% increase). And, as highlighted by DOJ, “[t]he government and whistleblowers were party to 543 settlements and judgments, the highest number of settlements and judgments in a single year.”[1]
This increase reflects DOJ’s laser-like focus on FCA enforcement. Last year, the government initiated 500 cases based on referrals or investigations, as opposed to qui tam matters. This far surpasses the prior record, set in 1987, of 340 government‑initiated cases, and outstrips the 305 matters opened the year before. Simply put, DOJ is aggressively seeking possible claims on its own initiative.
Historically, the vast majority of FCA recoveries have come from cases where DOJ initiated the case or intervened. Presumably, this portends significant FCA recoveries in years to come.
Number of FCA New Matters, Including Qui Tam Actions
Source: DOJ “Fraud Statistics – Overview” (Feb. 22, 2024)
B. TOTAL RECOVERY AMOUNTS
In FY 2023, the total dollars recovered through FCA cases (just shy of $2.7 billion) represented a significant increase over the previous year ($2.2 billion), though it remained far short of 2021’s most recent highwater mark ($5.7 billion).
Importantly, 2023 saw a return to normalcy in terms of the percentage of recoveries in which the United States either intervened or initiated the case. DOJ’s decision on whether to intervene historically has been a critical inflection point in cases—and one that strongly predicts whether a case will be successful. In short, DOJ is good at picking “winners.” FY 2022 presented a stark anomaly where more than half the FCA recoveries recorded (54%) stemmed from cases brought by a relator without the support of DOJ. This past year, FY 2023, 16% of recoveries came from qui tam actions in which the government declined to intervene, generally consistent with the overall trend and similar to numbers most recently seen in 2015 and 2017. Even that percentage, though, demonstrates the increase in the relators’ bar taking these cases into discovery and obtaining recoveries in recent years. Since 2014, relators have recovered a total of approximately four times more in FCA cases than in all other years since 2000 combined.
Assuming this indicator remains roughly consistent, we may soon see recoveries surpassing the record set in 2014, given DOJ’s aggressive initiation of cases in FY 2023.
Settlements or Judgments in Cases Where the Government Declined Intervention as a Percentage of Total FCA Recoveries
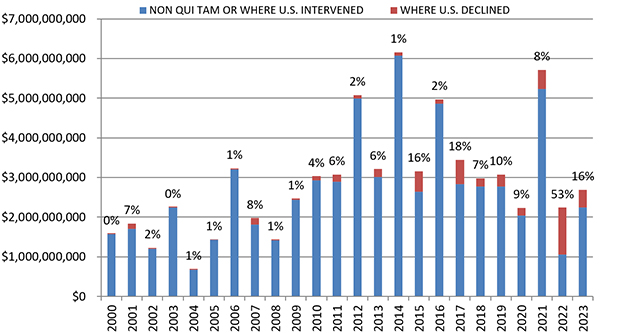
Source: DOJ “Fraud Statistics – Overview” (Feb. 22, 2024)
C. FCA RECOVERIES BY INDUSTRY
The breakdown of FCA recoveries by industry shifted marginally in 2023. Consistent with existing trends, healthcare cases accounted for the lion’s share of recoveries—68%, equating to more than $1.8 billion. That, however, represents the lowest portion of FCA recoveries since 2017 (then 63%). This shift is largely due to increased recoveries related to Department of Defense (DOD) procurement, which made up 21% of recoveries in 2023, equating to over $550 million. The remaining 12% of recoveries (nearly $320 million) were from cases involving other industries.
Regarding the healthcare-related claims, DOJ touted its cases alleging Medicare Advantage fraud, unnecessary services and substandard care fraud, claims related to the opioid epidemic, and unlawful kickbacks. Beyond those cases, DOJ emphasized its enforcement efforts targeting government defense contractors, as well as COVID-19 and cybersecurity fraud.[2]
FCA Recoveries by Industry
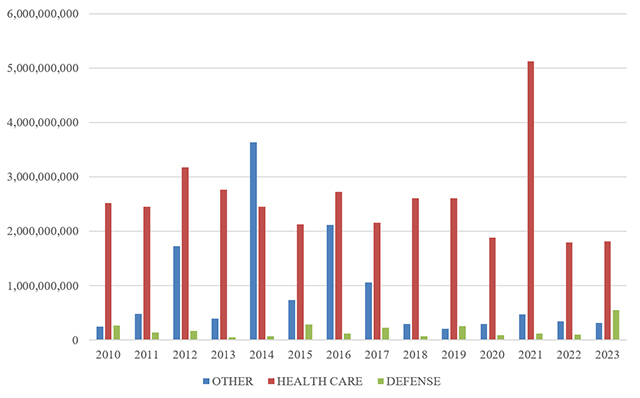
Source: DOJ “Fraud Statistics – Health and Human Services”; “Fraud Statistics – Department of Defense”; “Fraud Statistics – Other (Non-HHS and Non-DoD)” (Feb. 22, 2024)
II. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE SECOND HALF OF 2023
A. HEALTHCARE AND LIFE SCIENCE INDUSTRIES
- On July 13, a dermatology practice which operates 13 clinics in southeast Tennessee and north Georgia, and one of its dermatologists, agreed to pay $6.6 million to resolve allegations that they violated the FCA by overbilling federal healthcare programs for various dermatological surgeries and procedures. The government alleged that the practice and the physician falsely claimed that both the surgery and pathology portion of procedures were conducted by the dermatologist, when in fact certain portions were conducted by other individuals, and that the defendants regularly billed Medicare in a way that improperly bypassed Medicare’s “multiple procedure reduction rule.” As part of the settlement, the practice entered into a corporate integrity agreement (CIA) with the Department of Health and Human Services Office of the Inspector General (HHS-OIG), which focuses on the practice’s continuing obligation to properly bill and submit reimbursement claims to government payors. The settlement resolved a suit brought by a qui tam relator; the relator will receive $1.3 million of the settlement amount.[3]
- On July 14, an electronic health record (EHR) technology vendor agreed to pay $31 million to settle allegations that it violated the FCA and the federal Anti-Kickback Statute (AKS). The vendor allegedly misrepresented its software’s capabilities and falsely obtained certification from HHS under a program that grants incentive payments to incentivize healthcare providers that adopt EHR The vendor also allegedly provided unlawful remuneration to customers for referrals, including sales credits and tickets to sporting and entertainment events. This settlement is the latest in a series of resolutions with EHR vendors, including a $45 million settlement from late 2022 that we covered in our Year-End 2022 FCA Update. The settlement resolved a qui tam suit brought by two relators from a facility that used the company’s EHR software; the relators will receive nearly $5.6 under the settlement agreement.[4]
- On July 31, a Maine-based Medicare Advantage organization agreed to pay approximately $22.5 million to resolve allegations that it submitted inaccurate diagnosis codes for its enrollees, thereby increasing its reimbursements from Medicare. The government alleged that the provider reviewed the charts of its Medicare Advantage beneficiaries to identify additional diagnosis codes and then submitted those codes to Medicare although they were not supported by the patients’ medical records. The settlement resolved a qui tam suit brought by a former employee, who will receive approximately $3.8 million in the settlement.[5]
- On August 1, two pharmacy companies and their respective owners agreed to pay over $3.5 million to resolve allegations that they violated the FCA by billing Medicare for medications that were not actually dispensed. The companies also agreed to a five-year exclusion from participation in federal healthcare programs, surrendered their Drug Enforcement Agency (DEA) Certificates of Registration, and ceased operations.[6]
- On August 1, a clinical laboratory and its owner agreed to pay $5.7 million to settle an outstanding FCA judgment against them. An initial judgment of approximately $30.6 million was entered against the laboratory and owner in 2018 after a court found that the lab knowingly submitted false claims to Medicare for travel reimbursements. In particular, the court concluded that the lab billed Medicare for lab technician travel when specimens were not accompanied by technicians and billed travel for each specimen when groups of specimens were transported together. Due to the defendants’ inability to pay the original judgment in full, the laboratory and owner agreed to pay the new $5.7 million settlement over a period of five years. The settlement resolved a qui tam suit brought by the lab’s competitor, who will receive $1.3 million of the settlement. The settlement imposes future payment obligations in the event that certain contingencies transpire in relation to the laboratory owner’s income.[7]
- On August 10, a Florida-based durable medical equipment supply company agreed to pay $29 million to settle allegations that it fraudulently claimed reimbursement from Medicare and Medicare Advantage Plans for rental payments for oxygen equipment in excess of the 36-month cap on reimbursement. The settlement agreement stated that approximately $12.6 of the settlement amount constituted restitution. In conjunction with the DOJ settlement, the company also entered into a five-year CIA with HHS-OIG, which requires the company to undertake various compliance measures and to retain an independent compliance expert to review the company’s compliance program. The DOJ settlement resolved a qui tam suit brought by former employees, who will receive approximately $5.7 of the settlement amount.[8]
- On August 24, a Michigan pain management doctor and two pain center entities he owned and operated collectively agreed to pay $6.5 million to resolve a variety of FCA allegations. The government alleged that the doctor and entities billed Medicare and Medicaid for excessive and medically unnecessary urine drug tests irrelevant to patient treatment, additional laboratory charges not separately billable from the urine drug tests, routine moderate sedation services not actually required for interventional pain management procedures, and medically unnecessary or otherwise non-reimbursable back braces. The settlement resolves claims in two qui tam lawsuits, and the relators will collectively receive approximately $1.3 million of the settlement amount.[9]
- On August 30, a California company that operates multiple healthcare providers agreed to pay $5 million to resolve claims that it violated the FCA and the California FCA by causing the submission of false claims to California’s Medicaid program. The government alleged that the company billed for unallowable and/or inflated costs for services it provided to “Adult Expansion” population members (as defined under the Affordable Care Act). This settlement is the latest in a series of resolutions related to the Medicaid Adult Expansion program in California, through which the United States has recovered a total of $95.5 million. The allegations underlying the August 30 settlement stemmed from a qui tam suit by a former medical director, who will receive approximately $950,000 as his share of the recovery.[10]
- On September 13, a Texas company that managed and operated dermatology practices, surgical centers, and pathology laboratories across the United States agreed to pay approximately $8.9 million, including approximately $5.9 million in restitution, to resolve self-reported claims of potential violations of the FCA, the AKS, and the Stark Law. The government alleged that former senior managers of the company offered to increase the purchase price of 11 dermatology practices in exchange for agreements to refer laboratory services to affiliated entities after the acquisition. The government credited the company for self-reporting the alleged conduct at a time when the government was unaware of it. The settlement agreement itself, however, is not publicly available, and the press release announcing the settlement does not specify how much the cooperation credit reduced the amount the government otherwise would have sought to recover.[11]
- On September 15, a cardiac diagnostics company and its founder-owner agreed to pay approximately $4.5 million to resolve allegations that they paid physicians millions of dollars in the form of rent payments and referral fees to induce them to refer patients to the diagnostics company in violation of the AKS and the FCA. As part of the settlement, the government, the company and its founder-owner entered into a consent judgment for $64.4 million, which the government can seek to enforce if the required settlement payments are not made. The settlement agreement resolves a qui tam suit but does not specify the relator’s share of the recovery.[12]
- On September 30, a Connecticut-based healthcare and insurance company agreed to pay approximately $172 million to resolve allegations that it submitted, and failed to withdraw, inaccurate and untruthful diagnosis codes for its Medicare Advantage Plan enrollees. The government alleged that, as part of the company’s “chart review” program to identify all medical conditions that the charts supported and to assign the beneficiaries diagnosis codes for those conditions, the company added diagnosis codes that the patients’ healthcare providers had not included to inflate the payments the company received from the Centers for Medicare and Medicaid Services (CMS). In addition, the government alleged that the company failed to withdraw false diagnosis codes and repay CMS, including where the company itself added the codes and where providers included them initially and the company’s review did not support the use of the codes. Along with the settlement, the company entered into a five-year CIA with HHS-OIG, which requires (among other things) an independent review organization to audit the company with a focus on risk adjustment data.[13]
- On October 2, a Delaware-based specialty pharmacy and its CEO agreed to pay a total of $20 million to resolve FCA allegations premised on alleged AKS violations. Specifically, the government alleged that the company improperly waived Medicare and TRICARE patients’ copayments to induce patients to purchase the company’s services and specialty drugs, and that the company gave kickbacks to physicians to induce referrals in the form of gifts, dinners, and free administrative and clinical support services. The settlement stemmed from a qui tam suit brought by two former employees of the company, who together will receive approximately $4 million of the recovery.[14]
- On October 2, a California-based provider of genomic-based diagnostic tests agreed to pay $32.5 million to resolve allegations that it violated the FCA by improperly billing Medicare for the company’s principal laboratory test. In particular, the government alleged that the company violated the Medicare “14-Day Rule,” which governs reimbursement for laboratory tests for patients discharged after hospital stays. The settlement resolved two qui tam actions brought by two relators, who collectively will receive a share of approximately $5.7 million of the settlement.[15]
- On October 10, an Illinois-based cardiac imaging company and its founder agreed to pay approximately $85.5 million in an FCA resolution premised on alleged violations of the AKS and the Stark Law. The government alleged that the company paid above fair market value fees to cardiologists to supervise certain scans for patients the cardiologists referred to the company as improper referral; the fees allegedly included amounts for time that the cardiologists spent off-site or attending to other patients as well as for services beyond supervision that were not actually provided. The government also alleged that the company knowingly relied on a consultant’s fair market value analysis that was based on inaccurate information about the relevant services and that the consultant that provided the analysis later disclaimed. The company also entered into a five-year CIA with HHS-OIG, which imposes (among other obligations) an annual risk assessment and the retention of an independent review organization. The FCA settlement resolves a qui tam action brought by one of the company’s former billing managers, whose share of the government’s recovery had not been determined at the time the settlement was announced.[16]
- On October 17, multiple Michigan inpatient and hospitalist entities agreed to pay approximately $4.4 million to resolve allegations they violated the FCA by billing for services for beneficiaries located in Michigan and Indiana that were not rendered to the Michigan-based beneficiaries, permitting doctors to regularly bill impossible days of services, and by upcoding medical services (using more expensive billing codes than the codes corresponding to the services provided). The settlement resolves claims in two qui tam suits, with relators receiving approximately $767,000 of the settlement.[17]
- On October 30, a drug manufacturer and its founder agreed to pay at least $3.8 million and up to $50 million to resolve allegations that the company knowingly underpaid quarterly rebates to Medicaid programs for one of the company’s drugs. The government alleged that the drug manufacturer paused manufacturing of an acquired drug and later relaunched it as a reformulation (despite not changing any active ingredients) with a price increase of more than 400%. However, the manufacturer allegedly refused to pay the larger Medicaid rebate invoices tied to the price increase. The settlement amount is tied to certain financial contingencies.[18]
- On November 8, an eastern Kentucky hospital system and one of its physicians agreed to collectively pay approximately $3 million to resolve allegations that they violated the FCA by submitting claims for non-covered services to Medicare and Kentucky Medicaid. The government alleged that the hospital and physician billed, or caused to be billed, federal healthcare programs for reimbursement of services without the requisite documentation to support medical necessity of those services. The matter arose from the hospital system’s voluntary self-disclosure of the claims. The government stated explicitly that its recovery was limited to 1.5 times the amount of monetary loss caused by the alleged false claims, which is consistent with DOJ’s cooperation credit policy for FCA cases.[19]
- On November 9, DOJ announced that a healthcare management company, its executive, and six skilled nursing facilities agreed to a consent judgment in the amount of approximately $45.6 million to resolve claims that they violated the FCA and the AKS. The government alleged that under the direction and control of the management company and its executive, the skilled nursing facilities entered into medical directorship agreements that purported to compensate physicians for administrative services but actually provided kickbacks for physicians that referred patients to the skilled nursing facilities. The consent judgment calls for scheduled payments for each defendant based on their ability to pay, and the settlement contains a series of covenants by the government not to enforce the consent judgment as to certain assets, combined with provisions for increasing the payments owed by the defendants in the event of certain financial contingencies. In connection with the settlement agreement, the management company, one of the executives, and one of the skilled nursing facilities entered into a five-year CIA with HHS-OIG.[20]
- On December 6, a Pennsylvania-based company and its Illinois-based subsidiary agreed to pay more than $14.7 million to resolve allegations that they violated the FCA by knowingly submitting claims to federal healthcare programs for more expensive types of remote cardiac monitoring than what physicians had intended to order or that were medically necessary. According to the government, the companies ignored requests by physicians for types of monitoring that carried lower reimbursement rates than what the companies ended up billing. The settlement agreement resolved two qui tam actions brought by, respectively, an individual employee of one of the company’s customers and by an LLC. The individual will receive $2.3 million of the settlement share, and the LLC will receive approximately $270,000.[21]
- On December 19, a healthcare network agreed to pay $345 million to resolve allegations that it violated the FCA by knowingly submitting claims to Medicare for services that were referred to the network in violation of the Stark Law. In particular, the government alleged that senior management employed physicians and paid them above fair market value and in a way that took account of the volume of the physicians’ referrals to the network. The network allegedly continued this conduct despite warnings from a compensation valuation firm that the physician salaries exceeded fair market value. In connection with the settlement, the network entered into a five-year corporate integrity agreement with HHS-OIG. The settlement resolved a qui tam lawsuit brought by the network’s former Chief Financial and Chief Operating Officer, whose share of the recovery, according to the government “ha[d] not yet been determined” as of the time of the press release. The settlement agreement resolves only the allegations in the government’s partial complaint-in-intervention; as of this writing, the relator continues to pursue FCA claims premised on AKS allegations.[22]
- On December 20, a hospital operator agreed to pay $2 million, and to make additional payments in the event of certain contingencies, to resolve claims that the center violated the FCA. The government alleged that the center falsely claimed cost outlier payments—supplemental reimbursements by Medicare and TRICARE that aim to incentivize treatment for patients whose cost of care in an inpatient setting is particularly high. As part of the alleged conduct, the company improperly inflated its charges for inpatient care while underreporting charges on the cost reports it submitted to the government, and concealed an obligation to return outlier payments to which it was not entitled. The government also alleged that the company double‑billed the government for COVID-19 tests. The settlement resolved a qui tam suit brought by a former employee, who received approximately $300,000 of the settlement.[23]
- On December 21, a pharmaceutical company agreed to pay $6 million to resolve allegations that it violated the FCA by paying kickbacks in exchange for prescriptions. Specifically, the government alleged that the company knowingly paid for free genetic testing, as well as the associated fees; according to the government, the company knew the tests had to be positive for a certain genome in order for insurers to pay for the company’s medication. The settlement resolved a lawsuit brought by a qui tam relator, who will receive approximately $1.1 million of the federal settlement amount.[24]
- On December 21, a Missouri urgent care provider agreed to pay $9.1 million to settle allegations that it violated the FCA by submitting claims for physician services that actually were performed by non-physician practitioners. The settlement resolved allegations that the provider both upcoded billing for patient visits and submitted upcoded visit claims for COVID-19 vaccinations and patient care. The DOJ press release notes that the company “fully cooperated in the investigation,” but it is not clear whether DOJ awarded any cooperation credit on that basis. The press release did note that the company had voluntarily self‑disclosed separate conduct—the payment of bonuses to physicians in part based on the volume or value of referrals—to HHS-OIG in March 2021.[25]
- On December 22, a Pennsylvania manufacturer of durable medical equipment agreed to pay $2.5 million to resolve allegations that it violated the FCA by giving kickbacks to sleep laboratories. The government alleged that the manufacturer gave the laboratories free diagnostic sleep-respiratory disorder masks to induce prescriptions or referrals for masks the company manufactured for treatment of sleep disorders.[26]
B. GOVERNMENT CONTRACTING AND PROCUREMENT
- On July 21, a consulting firm agreed to pay approximately $377 million to resolve allegations that from 2011 to 2021 it violated the FCA by improperly billing unrelated or disproportionate costs to its government contracts. In particular, the government alleged that the firm billed the government for costs that were unallowable or that should have been allocated to commercial contracts instead of to government contracts. The settlement is the largest, by dollar value, since our 2023 Mid-Year Update, and is a rare example of an FCA settlement based on alleged violations of the federal cost accounting standards (CAS). The settlement resolved a qui tam suit brought by a former employee, who will receive almost $70 million of the settlement.[27]
- On July 21, two government contractors agreed to pay a total of $7 million to resolve allegations that they violated the FCA by falsely representing what methodology they used to measure customer satisfaction on certain government websites. The government alleged that the contractors were awarded a five-year contract with the Federal Consulting Group (a part of the U.S. Department of Interior) with the understanding that the company would measure customer satisfaction using the American Customer Satisfaction Index’s (ASCI) methodology, but that the company instead used a different methodology. The settlement resolves claims in a qui tam lawsuit brought by two relators, who will receive a total of $1.5 million of the settlement amount.[28]
- On August 4, an electronic connector manufacturing company agreed to pay approximately $18 million to settle allegations that it violated the FCA by submitting false claims for electrical connectors to the U.S. government and military. The company allegedly submitted claims for reimbursement of the connectors that did not meet the testing and manufacturing specifications required for the claims to be eligible for reimbursement.[29]
- On September 5, a New Jersey-based company paid approximately $4.1 million to resolve claims that it violated the FCA by failing to satisfy certain required cybersecurity controls in connection with information technology services provided to federal agencies. Specifically, the government alleged that the company failed to implement three required cybersecurity controls for Trusted Internet Connections with respect to General Services Administration (GSA) contracts from 2017 to 2021 within an internet protocol service it provided to federal agencies. The claims stemmed from a written self-disclosure of potential issues by the company, submitted to the GSA’s Office of Inspector General. The settlement agreement states that the company received credit for disclosure, cooperation, and remediation; although it does not specify the amount of the credit, it does detail certain cooperation and remediation steps the company took, such as identifying responsible individuals, disclosing facts it had gathered and attributing them to specific sources, assisting in damages calculations, and imposing employment consequences for responsible individuals.[30]
- On September 15, a Pennsylvania-based research and engineering services provider, agreed to pay $4.4 million to settle allegations that it violated the FCA by knowingly double billing for labor and material costs in relation to contracts with the U.S. Navy. The settlement agreement specifies that $2.1 million of the settlement amount constitutes restitution, and notes that there was a parallel administrative case that arose out of government audits of the relevant contracts and that the parties had agreed in principle to settle. It does not appear that there was a qui tam case underlying the settlement.[31]
- On September 28, a major military aircraft manufacturer agreed to pay $8.1 million to resolve allegations that it violated the FCA by failing to adhere to critical manufacturing requirements in the production of composite parts for certain aircraft sold to the United States military. The government alleged that the manufacturer failed to conduct routine checks and surveillance of machines used to cure certain composite parts, and that the manufacturer did not maintain required documentation concerning periodic testing of those machines. The settlement resolved a qui tam action by three whistleblowers who worked at the company’s manufacturing facility producing the composite parts; together they will receive approximately $1.5 million of the settlement.[32]
- On November 20, a Virginia-based tactical gear and equipment company agreed to pay nearly $2.1 to settle allegations that it submitted false claims in connection with the sale of “American-made” products that were actually manufactured in foreign countries in violation of the Trade Agreements Act and the Berry Amendment’s requirement that certain items purchased by DOD be 100% domestic in origin. The settlement resolved a qui tam suit brought by an employee, who will receive an unspecified portion of the settlement amount.[33]
C. OTHER
- On August 15, a Florida real estate broker and his companies agreed to pay $4 million to resolve FCA allegations that they knowingly provided false information in support of multiple Paycheck Protection Program (PPP) and Economic Injury Disaster Loan Program (EIDL) loans. The government alleged that the broker submitted false and fraudulent applications and documents, including false tax documents and employee wage reports, to obtain four EIDL loans and 14 PPP loans. The government also claimed that he submitted false and fraudulent forgiveness applications wherein he falsely certified that the entire loan amounts were used to pay eligible business costs.[34]
- On September 28, a Florida-based automotive company agreed to pay $9 million to resolve allegations that it violated the FCA by knowingly providing false information in support of a PPP loan application it submitted. The government contended that the company certified it was a small business and had fewer than 500 employees, making it eligible for PPP funds designated for “small business concerns” under the CARES Act. According to the government, the company in fact had over 3,000 employees, it knew when it made the certifications that it was ineligible for the loan program, and the government later forgave the loan. The settlement resolved claims brought in a qui tam lawsuit, and the relator will receive approximately $1.6 million of the recovery amount.[35]
- On October 31, an energy company agreed to pay $16 million to resolve allegations that it under-reported and under-paid natural gas royalties owed to the United States under administrative regulations for natural gas exploration. The government contended that the company knowingly deducted the costs of placing natural gas in marketable condition (which companies must do at no cost to the government) from the royalties it owed the government, knowingly deducted the costs of transporting carbon dioxide from the royalties, and knowingly failed to pay royalties on carbon dioxide. The settlement also resolved ongoing Department of Interior administrative proceedings regarding the same alleged conduct.[36]
- On November 1, a restaurant chain with locations in New York and Arizona and its owner agreed to pay $2 million to resolve allegations that they violated the FCA by falsely certifying that the restaurant chain was eligible to receive a Restaurant Revitalization Fund (RRF) grant in the amount of $928,554. Specifically, the government alleged that by falsely certifying that the restaurant chain did not have more than 20 locations, when in fact it had 21, the restaurant chain and its owner falsely claimed eligibility for the RRF grant. The settlement resolved a complaint filed by a qui tam relator whose share of the settlement will be $200,000.[37]
- On December 5, a Dallas-based importer of industrial products and two Chinese companies agreed to pay approximately $2.5 million to resolve allegations that they violated the FCA by submitting false invoices for customs valuations, which in turn resulted in lower values for the imported goods and lost customs revenue. The settlement resolved a qui tam suit brought by two relators, who received a $500,000 share as part of the settlement agreement.[38]
- On December 7, a New Jersey-based public relations firm agreed to pay nearly $2.3 million to settle allegations that the company violated the FCA by wrongfully taking a loan from the PPP. The United States contended that the company knowingly applied for and received a $2 million PPP loan, despite the fact that it was ineligible to receive the funds because it was a required registrant under the Foreign Agent Registration Act. The company allegedly later sought and received forgiveness for the total loan value. The settlement agreement states that the government considers approximately $2.1 million of the $2.3 million settlement amount to be restitution. The qui tam relator who brought the original lawsuit will receive $229,000, or 10%, of the recovery amount.[39]
- On December 11, a Texas‑based roofing company agreed to pay $9 million to resolve allegations that it violated the FCA by falsely certifying that eight of its affiliate companies were eligible to receive PPP loans in the amount of $6.7 million, which were all later forgiven in full. The government alleged that by improperly claiming to have fewer than 500 employees, each applicant falsely represented that it was qualified as a small business eligible to receive a loan, when the applicants’ affiliations with each other meant that they collectively had more than 500 employees. The settlement resolved a lawsuit brought by a qui tam relator who will receive $1 million of the settlement amount.[40] The law firm that represented the relator characterized it in a blog post as a “data miner” that “analyzes PPP loan data for prospective cases.”[41]
III. LEGISLATIVE AND POLICY DEVELOPMENTS
A. FEDERAL POLICY AND LEGISLATIVE DEVELOPMENTS
1. DOJ’s Cooperation Credit Policy, Several Years On
In the aggregate, FCA resolutions afford a fairly clear window into DOJ’s programmatic enforcement priorities. While any given settlement agreement’s level of detail regarding the covered conduct is often vigorously negotiated, agreements—and the press releases that announce them—typically contain enough high-level information about the nature of the government’s allegations for other companies in various industries to identify the government’s focus areas.
The government’s approach to awarding cooperation credit in FCA cases is markedly less transparent. In May 2019, DOJ issued a policy—now codified at Section 4-4.112 of the Justice Manual—regarding the circumstances under which such credit could be awarded.[42] At the core of the policy are voluntary disclosure, cooperation in the government’s investigation, and remediation.[43] In announcing the policy, DOJ stated that “[m]ost frequently, cooperation credit will take the form of a reduction in the damages multiplier and civil penalties,” and that DOJ “may publicly acknowledge the company’s cooperation.”[44] However, beyond that general statement, and a statement in the policy that cooperation credit cannot result in a defendant paying less than single damages, the policy said precious little about how much cooperation credit DOJ would award in various circumstances. (Gibson Dunn’s 2019 analysis of the policy provided further details on the policy and the significant discretion it granted to the government.)
The triad of disclosure, cooperation and remediation described in the FCA policy is a familiar one. In the criminal sphere, DOJ has made these same three concepts the centerpiece of its enforcement regime—not only from the standpoint of whether and how much cooperation credit to award, but also in terms of what type of resolution vehicle to use.[45] In the criminal enforcement context, however, DOJ tends to be more explicit about how much cooperation credit it awards and the factors that lead it to do so.
For example, several of DOJ’s new voluntary disclosure policies clarify that for a company that has made a qualifying self‑disclosure, DOJ will seek penalties of no more than 50% of the applicable criminal penalties, if the government determines criminal penalties are necessary.[46] These policies also make clear that companies that meet the policies’ criteria for disclosure, cooperation and remediation will not face guilty pleas absent aggravating factors.[47] Such policies also go beyond general pronouncements, and deal with more specific types of fact patterns that companies often face—the most notable example being DOJ’s recent “safe harbor” policy for companies that make voluntary self-disclosures regarding misconduct discovered in the course of mergers and acquisitions.[48] In the text of specific resolution agreements, moreover, DOJ frequently “shows its work” by explaining how much cooperation credit it is awarding and why. For example, in a recent deferred prosecution agreement with a commodities company related to alleged U.S. Foreign Corrupt Practices Act violations, the government explicitly awarded credit for cooperation efforts but not for voluntary disclosure, and stated that the company was receiving a 15% discount off the bottom end of the applicable U.S. Sentencing Guidelines penalty range.[49]
By contrast, nearly five years on from the codification of DOJ’s FCA cooperation credit policy, it is difficult to discern how DOJ is assessing the forms of cooperation and remediation the policy deems relevant, and to what effect in terms of settlement amounts. Before 2023, DOJ seldom invoked the policy as having affected the terms of a settlement when announcing resolutions. Although certain resolutions from 2023 reflect a possible shift toward more frequent discussion of the policy and provide valuable details about its application in practice, the statements DOJ is making continue to provide more questions than answers. Several examples from 2023 bear this out:
- In one of the year’s notable cybersecurity-related FCA resolutions, DOJ “acknowledged that [the company] took a number of significant steps entitling it to credit for cooperating with the government.”[50] These included a written self-disclosure to the GSA after the company learned of the relevant issues; “an independent investigation and compliance review of the issues and . . . multiple detailed supplemental written disclosures” to GSA; identification of responsible individuals to DOJ; disclosure of facts the company uncovered in its investigation, including by attributing the facts to specific sources; assistance to DOJ in the analysis of potential damages; and “prompt and substantial remedial measures” such as compliance enhancements, “substantial capital investments” in compliance initiatives, and employment consequences for responsible individuals.[51] The agreement, however, spends less than three lines stating that the company received cooperation credit; it does not specify which if any of the company’s efforts carried more weight than others in the government’s determination to award cooperation credit. The agreement did identify the portion of the amount that the government considered to be restitution; assuming this reflects DOJ’s views of single damages, then the total settlement amount was approximately 1.5 times the alleged single damages.[52]
- In another resolution involving a hospital system, DOJ did not publish the settlement agreement itself, but explicitly stated in the press release announcing the settlement that “[b]ecause the company self-reported the conduct to the government, it was able to resolve its False Claims Act liability for only 1.5 times the amount of monetary loss caused by its false claims.”[53]
The second example above suggests that voluntary self‑disclosure may be the engine of the cooperation credit analysis. Yet without more explicit statements from DOJ as to how it views companies’ disclosure, cooperation and remediation efforts, it is difficult to know which factors are ultimately responsible for any given award of cooperation credit.
On another level, while both examples above suggest that settlement at 1.5 times single damages is within reach for companies that satisfy DOJ’s policy, another resolution from 2023 awarded credit under the policy but reflected a reduction to only 1.75 times single damages. Further, like other resolutions, this one did not state which aspects of the companies’ efforts led DOJ to think that further reductions were inappropriate.[54] At the same time, DOJ has been known to settle at 1.5 times single damages—or even less—without any mention of self-disclosure, thus raising questions around the incentives for self-disclosure in the first instance. For example, in October, a New Jersey public relations firm reached a settlement of FCA allegations related to PPP funds, and the settlement amount was approximately 1.1 times the government’s stated restitution figure.[55]
In short, while DOJ’s more frequent invocation of its cooperation credit policy is a welcome development, for the time being it has done little to answer the questions the policy itself left open regarding the value of disclosure, cooperation, and remediation. Time will tell whether future resolutions will continue the recent trend of explicitly noting companies’ cooperation, and whether they will reflect a more detailed—and uniform—approach by DOJ to explaining how much cooperation credit it is awarding and why.
2. Civil Cyber-Fraud Initiative
Since announcing its Civil Cyber-Fraud Initiative in October 2021, DOJ has increasingly used the FCA to address cybersecurity concerns, and 2023 was no exception. The Civil Cyber‑Fraud Initiative uses the FCA to encourage disclosure and to hold accountable entities and individuals that put U.S. information or information systems at risk by knowingly providing deficient cybersecurity products or services, misrepresenting their cybersecurity practices or protocols, or violating obligations to monitor and report cybersecurity incidents and breaches.[56] Several recent cases highlight a growing trend of using the FCA to target government contractors that are required to meet certain cybersecurity requirements, even when no beach has occurred:
- In the cybersecurity-related FCA resolution mentioned above, the information services technology company paid $4.1 million to settle FCA allegations after self-disclosing potential issues with certain cybersecurity controls.[57] The company, which provides secure public internet connection capabilities to federal agencies, was contractually required to comply with the Office of Management and Budget’s Trusted Internet Connections initiative at all times. After identifying concerns with certain security controls that allegedly affected the company’s compliance with critical capabilities, the company self-disclosed the concern and implemented measures to remediate the issue.
- In a recently unsealed qui tam complaint stemming from the Civil Cyber-Fraud Initiative, a relator alleged that a university submitted false cybersecurity certifications to DOD. Despite making certifications of compliance with National Institute of Standards and Technology (NIST) requirements, the relator claims that the university failed to store controlling unclassified information in NIST-compliant applications, and replaced legitimate risk assessments with templates designed to simply “check the box.”[58]
These cases demonstrate that DOJ’s use of the FCA to pursue cybersecurity enforcement extends beyond commercial defense or cybersecurity-related contracts to any government agreement that includes representations about cybersecurity compliance. Healthcare companies should take note and pay particular attention to government contract provisions governing the storage, protection, and transmittal of protected health information and personal identifiable information, which may contain specific cybersecurity requirements or representations.
Cybersecurity enforcement will be an area to watch as it relates to self-disclosure in particular. In October 2023, DOD, the GSA, and NASA proposed a rule that would amend the Federal Acquisition Regulation (FAR) to require government contractors to disclose cybersecurity incidents within eight hours of discovering them, and to provide other periodic updates on efforts to remediate cybersecurity incidents.[59] Even though such disclosures to the government would not necessarily include information within the full scope of what it would consider relevant to possible FCA claims, the disclosures by definition will position the government to start investigating potential misconduct far closer in time to its occurrence than in other situations—even ones, such as the healthcare overpayment context, in which reporting to the government is required. The proposed FAR rule’s early reporting requirement could create additional incentives for federal contractors to quickly investigate and disclose potential FCA violations to DOJ, lest DOJ learn of the underlying cyber breaches too quickly for self-disclosure credit to be available. At the same time, it is possible DOJ will deem self-disclosure of potential FCA violations to carry less weight given that disclosure of the fact of a cyber incident would already be required by law. If the eight-hour disclosure provision remains in the FAR rule when it becomes final, it will be instructive to track the extent to which DOJ calibrates its application of the cooperation credit policy in the cyber context to focus on cooperation and remediation, as opposed to disclosure.
3. Other Federal Policy Developments
HHS-OIG Compliance Program Guidance
On November 6, 2023, HHS‑OIG released its new General Compliance Program Guidance (GCPG).[60] This document is designed to serve as a non-binding guide for healthcare entities, and includes guidance about compliance with the FCA and other applicable laws. The GCPG describes how the FCA in the healthcare context encompasses billing services or items to Medicare or Medicaid “where the service is not actually rendered to the patient, is already provided under another claim, is upcoded, or is not supported by the patient’s medical record.”[61] To ensure compliance with the FCA, the guidance recommends that entities take “proactive measures . . . including regular reviews to keep billing and coding practices up-to-date as well as regular internal billing and coding audits.”[62]
The guidance also outlines seven focus areas for corporate compliance programs, including policies and training, governance and reporting, risk assessments and audits, employment consequences, and responding to discoveries of misconduct.[63] In its discussion of risk assessments and auditing, the guidance makes clear that entities should put in place mechanisms for auditing the effectiveness of compliance controls, beyond simply auditing with an eye to identifying potential violations of law.[64] And the guidance emphasizes that an entity’s response to discovering misconduct should include self-disclosure to the appropriate government authority where “credible evidence of misconduct from any source is discovered and, after a reasonable inquiry, the compliance officer or counsel has reason to believe that the misconduct may violate criminal, civil, or administrative law.”[65] Notably, the guidance states that such disclosure should be made “not more than 60 days after the determination that credible evidence of a violation exists.”[66] It remains to be seen the extent to which this expectation ends up at odds with DOJ’s view of when an “obligation” to return healthcare overpayments arises under the Affordable Care Act (ACA) and the FCA, given that the ACA requires entities to return overpayments within 60 days of identifying them.[67]
HHS‑OIG has also signaled its intent to release industry segment-specific compliance program guidance in 2024 and to update the documents periodically.[68]
COVID-19 Enforcement
DOJ’s FCA enforcement efforts related to the COVID-19 pandemic are part of a broader landscape of civil and criminal enforcement initiatives to which DOJ has devoted significant resources over the last several years. In August 2023, DOJ provided an update on the efforts of its COVID-19 Fraud Enforcement Task Force.[69] According to DOJ, it had seized over $1.4 billion in COVID-19 relief funds as of that date, and had recently conducted a single coordinated enforcement effort involving 371 defendants and $836 million in relief funds, primarily from the Paycheck Protection Program, the Internal Revenue Service (IRS) Employee Retention Credit program, and Economic Injury Disaster Loans.[70]
B. STATE LEGISLATIVE DEVELOPMENTS
2023 saw states continue to expand the reach of their FCA statutes, some more aggressively than others. Most notably, as we discussed in our 2023 Mid-Year Update, New York became the first state to amend its FCA to cover persons who improperly fail to file a tax return in the state, obviating the need for the State or relators to show the person submitted an actual false “claim, record, or statement.” Connecticut also expanded the scope of its FCA statute to include claims relating to most state programs and benefits (although explicitly carving out tax-related claims), rather than only state-administered health and human services programs, as it had previously.[71]
More recently, New Jersey also amended its FCA statute. It did so specifically to qualify for the federal financial incentive that allows states to receive a ten-percentage-point increase in their shares of any amounts recovered under the FCA if the state’s laws meet certain specified criteria.[72] For a state to qualify for this incentive, HHS-OIG must determine that the state’s FCA is “at least as effective” as the federal FCA at facilitating qui tam actions.[73] With its bill, New Jersey implemented changes to bring the state’s law in line with HHS-OIG’s guidance. In addition to clarifying certain language and terminology to better align the statute with the federal FCA, the amendment expanded protections for relators by removing the bar preventing “an employee or agent of the State or a political subdivision from bringing an action based on information discovered in a civil, criminal, or administrative investigation or audit that was within the scope of the employee’s or agent’s duties or job description” and by expanding anti-retaliation protection to contractors and agents, beyond just employees.[74] With New Jersey’s amendment, there are now 23 state FCAs on HHS-OIG’s “approved” list and six on its “not approved” list.[75]
In Washington state, meanwhile, the legislature repealed a sunset provision that applied to whistleblower provisions, thus allowing qui tam actions to continue to be brought indefinitely.[76] The sunset provision was initially put in place to address the concern that the availability of qui tam actions would cause relators to indiscriminately file claims under the Washington FCA, but legislators found that this did not occur and the legislature’s Joint Legislative Audit and Review Committee unanimously recommended the bill repealing the sunset provision.[77]
IV. CASE LAW DEVELOPMENTS
A. The Third Circuit Weighs in on FCA Materiality Post-Escobar
The Supreme Court’s decision in Universal Health Servs., Inc. v. United States ex rel. Escobar, 579 U.S. 176 (2016), set forth a number of factors relevant to the potential materiality of a misrepresentation under the FCA. Among those factors are (1) whether the government has “designate[d] compliance with” the relevant “statutory, regulatory, or contractual requirement as a condition of payment”; (2) whether the alleged violation is “minor or insubstantial”; and (3) whether the government continued to pay claims “despite its actual knowledge that certain requirements were violated” or, instead, “consistently refuse[d] to pay claims in the mine run of cases based on noncompliance.” Id. at 194–95.
In late August, the Third Circuit addressed the interplay of these factors. In United States v. Care Alternatives, 81 F.4th 361 (3d Cir. 2023), the court reversed the district court’s grant of summary judgment to a defendant based on materiality. Relators in the case were former employees of Care Alternatives, a for-profit hospice provider. They alleged that Care Alternatives submitted Medicare claims even though patient records did not document hospice eligibility as required under 42 C.F.R. § 418.22(b)(2). Under that provision, for a patient to be eligible for hospice care paid by Medicare, a physician must certify that the patient is “terminally ill,” meaning that the physician has signed the certification with the knowledge that the patient’s medical record “‘support[s] the medical prognosis’ of terminal illness.” Id. at 366 (citation omitted). Relators alleged that Care Alternatives submitted claims that were accompanied by physician certifications of terminal illness, but that the patients’ records lacked sufficient clinical documentation “supporting that diagnosis.” Id. at 367.
The district court granted Care Alternatives’ first summary judgment motion based on a failure to show falsity; the Third Circuit reversed. (We covered the Circuit Court opinion in our 2020 Mid-Year Update.) Care Alternatives then filed a second motion for summary judgment. The district court again granted summary judgment to Care Alternatives, this time holding that relators had not established that the alleged misrepresentations to Medicare were material, given that the government continued to reimburse claims from Care Alternatives even after being made aware of the deficiencies in the underlying patient records.
The Third Circuit reversed and remanded, holding that the district court improperly assigned dispositive weight to a single factor under Escobar—that the government continued to reimburse despite knowing of the alleged clinical documentation deficiencies. The court concluded that a dispute of fact remained as to whether Care Alternatives’ alleged regulatory violations were “minor” or “went to the very essence of the bargain,” given that the parties contested the pervasiveness of the documentation errors, Care Alternatives’ awareness of its compliance problems, and whether the patients at issue were eligible for the Medicare hospice benefit. Id. at 370–72 (internal quotation marks omitted). And the court determined that, contrary to the district court’s opinion, a dispute of fact also remained as to whether the government ever had “actual knowledge” of the violation during the period in which it continued to reimburse Care Alternatives. Id. at 374–75. Noting that “relators are not required to conduct discovery on government officials to demonstrate materiality,” the court held that Care Alternatives had not met its burden of demonstrating an absence of dispute as to the timing of the government’s knowledge. Id. at 375.
B. The Fifth Circuit Overturns a Jury Verdict for the Government on Statute-of-Limitations Grounds
In United States v. Corporate Management, Inc., 78 F.4th 727 (5th Cir. 2023), a Medicare overbilling case, the Fifth Circuit heard an appeal following a nine-week jury trial that resulted in an approximately $10.8 million verdict for the government (roughly $32 million after trebling). Defendants appealed on a number of grounds, including that certain claims the government added when it intervened were untimely under the FCA’s statute of limitations. The relator filed the initial complaint in May 2007, alleging that Defendants had submitted false claims to Medicare, including by overbilling for supply costs. Id. at 734–35. The government did not intervene until September 2015. Id. at 735. In its complaint‑in‑intervention, the government added two claims, including a claim that Defendants “took advantage of Medicare’s 101% reimbursement rate” for critical access hospitals by setting up a sham “management fee” agreement between one such hospital and a management company owned by the hospital’s owner, which was used to improperly inflate salaries paid to the owner and his wife. Id. On appeal, Defendants argued that all claims accruing before September 2009, six years prior to the government’s complaint, were barred by the statute of limitations, and that the judgment should therefore be reduced to approximately $4.6 million. Id. at 741. The government argued that its claims related back to the relator’s original allegations that Defendants submitted fraudulent Medicare cost reports, or in the alternative, that the claims were viable in light of the FCA’s tolling period (which tolls the limitations period for up to three years “after the date when facts material to the right of action are known or reasonably should have been known by the official of the United States charged with responsibility to act in the circumstances”). Id.; 31 U.S.C. § 3731(b)(2).
The Fifth Circuit disagreed with the government on both arguments. It explained that to relate back, a new claim must be “tied to a common core of operative facts.” 78 F.4th 742 (internal quotation marks omitted). Whereas both the relator and the government alleged fraudulent cost reporting, the relator’s complaint contained no allegations regarding inflated salaries paid to the hospital owner and his wife. Id. at 743. Thus, rather than merely “add detail or clarify the claims on which it [was] intervening,” the government made new claims and sought to “‘fault [Appellants] for conduct different from that’ alleged by” the relator. Id. (internal quotation marks omitted). The Fifth Circuit further held that the government could not invoke the FCA’s three-year tolling provision, because there was evidence—in the form of a sealed government motion seeking an extension of the seal period—that an expert recommended in August 2011 that the government intervene in the case, and thus that by that time the government “likely did know” facts material to the case. Id. at 745 (emphasis in original). In reaching this conclusion, the court declined to decide whether DOJ or the relevant Medicare administrative contractor was the “official of the United States charged with responsibility to act in the circumstances” under the FCA’s tolling provision. Id.; 31 U.S.C. § 3731(b)(2).
C. The Ninth Circuit Imposes Limits on Calculation of Statutory Penalties and Upholds “Actual Damages” Framework
The FCA imposes a penalty for each violation of the statute, as well as “3 times the amount of damages which the Government sustains because of the act of” the defendant. 31 U.S.C. § 3729(a). In cases involving allegations that claims for payment were “tainted” by a defendant’s violation of an underlying contractual or regulatory requirement, FCA plaintiffs frequently seek penalties for every claim, and argue that the relevant goods or services were worthless to the government and that the proper measure of damages thus should be based on the full value of each claim.
In Hendrix ex rel. United States v. J-M Manufacturing Co., Inc., 76 F.4th 1164 (9th Cir. 2023), the Ninth Circuit reinforced important limitations on both of these theories. Relator and government entity plaintiffs in the case claimed that J-M violated the FCA by falsely representing that its PVC pipes were compliant with certain industry standards which measured compliance based on the material of the pipes and its performance under a series of tests, as well as the manufacturing process for the pipes. Under relevant standards, if the tested pipes are compliant, the manufacturer can claim compliance with those standards for pipes produced thereafter without additional testing, so long as the pipes are produced through “materially unchanged processes.” Id. at 1168.
During the first phase of a bifurcated trial, plaintiffs sought to prove that J-M continued to advertise its PVC pipes as compliant with industry standards after materially changing its manufacturing process from the pipes’ last compliance testing. At the end of Phase One, the jury found that J-M knowingly made materially false claims for payment because it represented in marketing materials that its pipes were uniformly compliant with industry standards. The jury made no findings regarding the physical longevity of any particular piece of pipe. During the damages phase of the trial, the district court granted judgment as a matter of law in favor of J-M on actual damages after the jury was unable to reach a verdict. The court awarded plaintiffs one statutory penalty per project at issue, and not—as the plaintiffs had sought—one penalty for each piece of pipe that had been stamped with the relevant industry standard. The court declined to impose damages, stating that the government had not established that the pipes were value-less.
The Ninth Circuit affirmed, ruling that the plaintiffs were not entitled to recover the entire PVC purchase price without a showing that the pipes had not operated as intended, and lacked all value in light of the jury’s Phase One findings. To the contrary, plaintiffs had successfully installed the pipes and used them for many years following installation without issue, and apparently without any plans to replace the pipes. To award damages in these circumstances, the Ninth Circuit held, would “impose a strict liability standard” without requiring proof of actual damages, and would “conflate[] ‘the materiality element of the FCA claim’ with ‘actual damages.’” Id. at 1174. In considering other cases in which the full contract price was awarded as damages, the court distinguished those cases as involving goods that “were either plainly unusable, not used, or returned.” Id.
The Ninth Circuit also rejected plaintiffs’ claims that statutory penalties should be awarded for each piece of PVC pipe purchased, rather than for each individual project. The court reasoned that the government entity plaintiffs “did not establish how much non-compliant pipe they received nor were they able to identify any specific piece of non-complaint pipe.” Id. at 1172.
The J-M case is an important reminder that there are limits on local, state, and federal governments’ ability to accumulate FCA damages merely because a regulatory violation preceded the provision of goods or services. Time will tell how closely other courts hew to the Ninth Circuit’s admonition that the materiality of a particular regulatory requirement does not automatically mean that goods or services provided after such a violation was committed were worthless.
D. The Tenth Circuit Clarifies a Prior Ruling on the FCA’s Retaliation Provision
In United States ex rel. Barrick v. Parker-Migliorini International, the Tenth Circuit clarified the extent to which an employer must be on notice that a relator is engaging in conduct protected by the FCA in order for the employer to be liable for retaliatory termination. 79 F.4th 1262 (10th Cir. 2023). Brandon Barrick was a senior financial analyst for PMI, who alleged FCA claims regarding two methods of beef distribution. First, according to Barrick, PMI exported beef to Costa Rica, which accepted beef subject to a lower (and therefore, cheaper) USDA testing standard, which would then be repackaged and sold to Japan, which required a higher (and more expensive) testing standard. Second, PMI was allegedly submitting beef to the USDA for testing, indicating that it was being sent to Moldova—when it was, in fact, being sent to Hong Kong, and, in turn, illegally smuggled into China. Id. at 1268–69.
Barrick alleged he had several conversations with PMI’s CFO regarding his concerns, and that the CFO confirmed that PMI was implementing these schemes and that they were illegal. Id. at 1268–69. Over the course of six months, Barrick allegedly cooperated with USDA, DOJ, and the FBI, including by recording several conversations with the CFO. See id. at 1271. Barrick alleged that one month after the FBI raided PMI’s offices, Barrick was terminated as part of a company-wide reduction in force of nine personnel. Id. at 1269. PMI claimed it did not learn of Barrick’s cooperation with the government until nearly two years later. Id.
The FCA prohibits retaliation for “lawful acts done by the employee . . . in furtherance of an action under this section or other efforts to stop 1 or more violations of this subchapter.” 31 U.S.C. § 3730(h)(1) (emphasis added). In 2022, the Tenth Circuit held that to show they were the victim of unlawful retaliation, a relator must show that (1) they were engaging in protected activity; (2) their employer had notice that they were engaged in protected activity; and (3) the employer terminated them because of their engagement in protected activity. Barrick, 79 F.4th at 1270 (citing U.S. ex rel. Sorenson, 48 F.4th 1146, 1158–59 (10th Cir. 2022)). In the Barrick case, PMI argued that to satisfy the notice prong of this standard, “Barrick was required to ‘convey a connection to the FCA.’” 79 F.4th at 1270. Relying on the FCA’s broad protection of “‘other efforts’ to stop [FCA] violations,” the court clarified that relators need not “say magic words, such as ‘FCA violation’ or ‘fraudulent report to the government to avoid payment,’ to put [employers] on notice.” Id. Rather, the person “must have conveyed to [the employer] that he was attempting to stop [the employer] from (1) engaging in fraudulent activity to avoid paying the government an obligation or (2) claiming unlawful payments from the government.” Id. at 1271. The employer “does not need to know the activity violates the FCA specifically.” Id. The court then upheld the jury’s findings that there was sufficient circumstantial evidence upon which the jury could have found PMI was aware that Barrick was engaging in protected conduct.
The Barrick case is significant because it means that, at least in the Tenth Circuit, the requisite nexus between an employee complaint and the FCA may be satisfied by evidence that the employee gave notice she was attempting to stop efforts to claim unlawful payments from, or efforts to unlawfully avoid making payments to, the government. That creates a tension with the text of the FCA’s anti-retaliation provision, which is specific to employee acts in furtherance of qui tam suits or of other efforts to stop a violation of the FCA in particular. Barrick seemingly left open the question of whether the employee herself must believe the conduct she is reporting violates the FCA specifically, or whether it is sufficient that the employee believe the conduct violates any of the myriad statutory, regulatory, and contractual requirements that are often used as the basis for FCA claims.
E. The Eleventh Circuit Reinforces a Strict Approach to Pleading Presentment Under Rule 9(b)
In some federal jurisdictions, including the Eleventh Circuit, to prevail on an FCA claim, “a relator must allege an actual false claim for payment that was presented to the government.” Carrel v. AIDS Healthcare Found., Inc., 898 F.3d 1267, 1277 (11th Cir. 2018) (internal quotation marks and emphasis omitted). In United States ex rel. 84Partners, LLC v. Nuflo, Inc., 79 F.4th 1353 (11th Cir. 2023), the Eleventh Circuit affirmed the district court’s dismissal of an FCA claim based on failure to allege presentment with the requisite particularity.
Relator—an entity called 84Partners, LLC, which included two former employees of a shipbuilding contractor and a pipe fitting manufacturer—brought a false-presentment FCA claim against their former employers, as well as a subcontractor and a pipe fitting distributor, based on the alleged installation of defective pipe fittings on nuclear submarines subsequently delivered to the Navy. Relator alleged that the manufacturer made defective parts and that the other defendants recklessly disregarded their obligations to inspect the parts before delivery or installation, resulting in at least 42 defective pipe fittings being installed on Navy vessels. The Navy made payments for all “allowable costs,” which included costs for parts installed on nuclear submarines, but relator failed to identify “any claim for payment submitted to the Navy that included any of the 42 [defective] parts.” Id. at 1357 (alteration added).
The district court dismissed the relator’s second amended complaint with prejudice, noting that, despite eight years of litigation and limited discovery, relator was still unable to state a claim for false presentment; the court also noted that relator had not requested leave to further amend its operative complaint. Id. at 1358. The Eleventh Circuit affirmed, concluding that although the “complaint allege[d] with particularity egregious underlying conduct,” it failed to “allege with particularity the actual submission of false claims—claims covering the 42 defective parts, or any other defective parts, that made it into submarines.” Id. at 1361. Notably, the government had filed an amicus brief in the Eleventh Circuit, in which it urged the court to eschew a requirement to plead actual false claims—arguing, among other things, that the requirement is a poor fit for cases in which the government is the “only buyer . . . and requests for payment are submitted to one potential government payer under readily-identifiable contracts.” Brief for United States as Amicus Curiae, United States ex rel. 84Partners, LLC v. Nuflo, Inc., No. 21-13673, at 23 (11th Cir. Jan. 20, 2022). The government attempted to contrast such fact patterns with healthcare cases, in which the claims submission process is more complex and involves entities beyond the government itself. See id. While the government made other arguments against the application of a strict Rule 9(b) standard, this explicit contrast between types of FCA cases—and the insinuation that a stricter Rule 9(b) standard may actually have a role to play in healthcare cases in particular—is an interesting window into how the government thinks about different FCA fact patterns.
F. The District of Massachusetts Sets the Stage for a Deepened Circuit Split over Causation in AKS-Predicated FCA Cases
Our 2023 Mid-Year False Claims Act Update discussed the deepening circuit split over the proper causation standard for AKS-predicated FCA claims. In brief, the Sixth Circuit and Eight Circuit have held that the AKS imposes a “but for” causation standard, see e.g., United States ex rel. Martin v. Hathaway, 63 F.4th 1043, 1052–53 (6th Cir. 2023); United States ex rel. Cairns v. D.S. Medical L.L.C., 42 F.4th 828 (8th Cir. 2022), whereas the Third Circuit has rejected a “but‑for” causation standard and instead determined that the FCA and AKS “require[] something less than proof that the underlying medical care would not have been provided but for a kickback.” United States ex rel. Greenfield v. Medco Health Solutions, Inc., 880 F.3d 89, 96 (3d Cir. 2018). Now, the First Circuit is also set to rule on this question after the district court granted interlocutory appeal in two cases with opposite holdings: United States v. Regeneron Pharms., Inc., No. CV 20-11217-FDS (D. Mass.) and United States v. Teva Pharms. USA, Inc., Civil Action No. 20-11548-NMG (D. Mass.).
In Teva, the government alleged that Teva caused the submission of false claims to Medicare through kickbacks it paid in the form of co-pay subsidies in connection with the sale of its multiple sclerosis drug, Copaxone. Both Teva and the United States filed motions for summary judgment on the issue of causation, among other issues. Teva argued that the government must prove “but-for” causation, citing Martin, 63 F.4th 1043 and Cairns, 42 F.4th 828. The government argued that the FCA only requires a “sufficient causal connection” between a kickback and a claim, citing Guilfoile v. Shields, 913 F.3d 178, 190 (1st Cir. 2019) and Greenfield, 880 F.3d 89. On July 14, 2023, the district court in Teva held that “[t]he government need not prove ‘but for’ causation,” and concluded that “[t]he government has established evidence of ‘a sufficient causal connection’ between Teva’s payments to CDF and ATF and the resulting Medicare Copaxone claims.” Teva, 2023 WL 4565105, at *3–4 (D. Mass. July 14, 2023). Thus, the Teva court joined the Third Circuit in rejecting the “but-for” causation standard. On August 14, 2023, the Teva court granted interlocutory appeal on the causation question, finding that the standard for causation is “a controlling question of law as to which there is substantial ground for difference of opinion and an immediate appeal may materially advance the ultimate termination of this litigation.” Teva, Order, Docket No. 235. (D. Mass. Aug. 14, 2023) (internal citations omitted).
In Regeneron, the government alleged that Regeneron improperly sent millions of dollars to an independent charitable foundation to subsidize patient co-pays for Eylea, a drug that treats neovascular (wet) age-related macular degeneration. Similar to the Teva case, both the government and the company filed motions for summary judgment on the causation standard. On September 27, 2023—months after the Teva court issued its ruling—the Regeneron court held that the appropriate standard for causation was “but for” causation and that “the factual evidence is sufficient to withstand summary judgment on the issue of causation.” Regeneron, 2023 WL 6296393, at *13 (D. Mass. Sept. 27, 2023). On October 25, 2023, the Regeneron court certified its decision for interlocutory appeal to the First Circuit on the same question as the Teva case, stating that “if both this matter and the Teva matter were to proceed to trial—and both trials are expected to be lengthy and complex—at least one of those trials would employ an incorrect causation standard, and thus waste considerable time and resources.” Regeneron, 2023 WL 7016900, at *1 (D. Mass. Oct. 25, 2023). The Regeneron court further reiterated that “the issue is one of national importance, as reflected in the split among the circuits as to the correct standard.” Id.
Both cases have been accepted by the First Circuit, but it has not yet ruled. That ruling seems likely to deepen the existing circuit split on the issue of causation. But it remains to be seen whether the addition of another Circuit-level decision will prompt the Supreme Court to weigh in where it has not done so to date. (In October the Court denied a certiorari petition in the Martin case.) This issue carries significant implications for FCA defendants, as exemplified by the $487 million jury verdict in May 2023 against a medical supply company in a case involving allegations of false claims caused by illegal kickbacks. (We covered this case in our 2023 Mid-Year Update.)
V. CONCLUSION
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2024 False Claims Act Mid-Year Update.
__________
[1] Press Release, U.S. Dep’t of Justice, False Claims Act Settlements and Judgments Exceed $2.68 Billion in Fiscal Year 2023 (Feb. 22, 2024), https://www.justice.gov/opa/pr/false-claims-act-settlements-and-judgments-exceed-268-billion-fiscal-year-2023 [hereinafter DOJ FY 2023 Recoveries Press Release].
[2] DOJ FY 2023 Recoveries Press Release.
[3] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Tenn., Dermatologist Agrees to Pay $6.6 Million to Settle Allegations of Fraudulent Billing Practices (July 13, 2023), https://www.justice.gov/usao-edtn/pr/dermatologist-agrees-pay-66-million-settle-allegations-fraudulent-billing-practices.
[4] See Press Release, U.S. Atty’s Office for the Dist. of Vt., Electronic Health Records Vendor NextGen Healthcare, Inc. to Pay $31 Million to Settle False Claims Act Allegations (July 14, 2023), https://www.justice.gov/usao-vt/pr/electronic-health-records-vendor-nextgen-healthcare-inc-pay-31-million-settle-false; United States ex rel. Markowitz et al. v. NextGen Healthcare, Inc., Case No. 2:18-cv-195 (D. Vt.), Settlement Agreement, https://www.justice.gov/opa/file/1305766/dl?inline.
[5] See Press Release, Dep’t of Justice, Martin’s Point Health Care Inc. to Pay $22,485,000 to Resolve False Claims Act Allegations (July 31, 2023), https://www.justice.gov/opa/pr/martins-point-health-care-inc-pay-22485000-resolve-false-claims-act-allegations.
[6] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Northeast Philadelphia Pharmacies and Their Owners Agree to Pay Over $3.5 Million to Resolve False Claims Act Liability (Aug. 1, 2023), https://www.justice.gov/usao-edpa/pr/northeast-philadelphia-pharmacies-and-their-owners-agree-pay-over-35-million-resolve.
[7] See Press Release, Dep’t of Justice, Clinical Laboratory and Its Owner Agree to Pay an Additional $5.7 Million to Resolve Outstanding Judgement for Billing Medicare for Inflated Mileage-Based Lab Technician Travel Allowance Fees (Aug. 1, 2023), https://www.justice.gov/opa/pr/clinical-laboratory-and-its-owner-agree-pay-additional-57-million-resolve-outstanding.
[8] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Wash., Lincare Holdings Agrees to Pay $29 Million to Resolve Claims of Overbilling Medicare for Oxygen Equipment in Largest-Ever Health Care Fraud Settlement in Eastern Washington (Aug. 28, 2023), https://www.justice.gov/usao-edwa/pr/lincare-holdings-agrees-pay-29-million-resolve-claims-overbilling-medicare-oxygen; see also Settlement Agreement, Case No. 2:21-cv-151-TOR (E.D. Wash.), https://www.justice.gov/usao-edwa/file/1311981/dl?inline.
[9] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Mich., Michigan Doctor to Pay $6.5 million to Resolve False Claims Act Allegations (Aug. 24, 2023), https://www.justice.gov/usao-edmi/pr/michigan-doctor-pay-65-million-resolve-false-claims-act-allegations.
[10] See Press Release, U.S. Dep’t of Justice, Office of Pub. Affairs, Health Care Provider Agrees to Pay $5 Million for Alleged False Claims to California’s Medicaid Program (Aug. 30, 2023),
https://www.justice.gov/opa/pr/health-care-provider-agrees-pay-5-million-alleged-false-claims-californias-medicaid-program https://www.justice.gov/opa/pr/health-care-provider-agrees-pay-5-million-alleged-false-claims-californias-medicaid-program.
[11] See Press Release, U.S. Atty’s Office for the Northern Dist. of Tex., Dermatology Management Company to Pay $8.9 Million to Resolve Self-Reported False Claims Act Liability (Sept. 13, 2023), https://www.justice.gov/usao-ndtx/pr/dermatology-management-company-pay-89-million-resolve-self-reported-false-claims-act.
[12] See Press Release, U.S. Atty’s Office for the Southern Dist. of N.Y., U.S. Settles False Claims Act Lawsuit Against Cardiologist and His Medical Practice for Paying Millions in Kickbacks for Referrals (Sept. 18, 2023), https://www.justice.gov/usao-sdny/pr/us-settles-false-claims-act-lawsuit-against-cardiologist-and-his-medical-practice.
[13] See Press Release, U.S. Dep’t of Justice, Cigna Group to Pay $172 Million to Resolve False Claims Act Allegations (Sept. 30, 2023), https://www.justice.gov/opa/pr/cigna-group-pay-172-million-resolve-false-claims-act-allegations.
[14] See Press Release, U.S. Dep’t of Justice, United States Settles Kickback Allegations with BioTek reMEDys Inc., Chaitanya Gadde and Dr. David Tabby (Oct. 2, 2023), https://www.justice.gov/opa/pr/united-states-settles-kickback-allegations-biotek-remedys-inc-chaitanya-gadde-and-dr-david.
[15] See Press Release, U.S. Atty’s Office for the Eastern Dist. of N.Y., Genomic Health Inc. to Pay $32.5 Million to Resolve Allegations Relating to the Submission of False Claims for Genomic Diagnostic Tests (Oct. 2, 2023), https://www.justice.gov/usao-edny/pr/genomic-health-inc-pay-325-million-resolve-allegations-relating-submission-false.
[16] See Press Release, U.S. Dep’t of Justice, Mobile Cardiac PET Scan Provider and Founder to Pay $85 Million to Resolve Allegedly Unlawful Payments to Referring Doctors (Oct. 10, 2023), https://www.justice.gov/opa/pr/mobile-cardiac-pet-scan-provider-and-founder-pay-85-million-resolve-allegedly-unlawful.
[17] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Mich., Hospitalist Companies Agree to Pay Nearly $4.4 Million to Settle False Claims Act Allegations (Oct. 17, 2023), https://www.justice.gov/usao-edmi/pr/hospitalist-companies-agree-pay-nearly-44-million-settle-false-claims-act-allegations#:~:text=(defendants)%20have%20agreed%20to%20pay,one%20day%2C%20and%20billing%20for.
[18] See Press Release, U.S. Dep’t of Justice, Drugmaker Nostrum and Its CEO Agree to Pay Up to $50 Million to Settle False Claims Act Claims for Underpaying Rebates Owed Under Medicaid Drug Rebate Program (Oct. 30, 2023), https://www.justice.gov/opa/pr/drugmaker-nostrum-and-its-ceo-agree-pay-50-million-settle-false-claims-act-claims.
[19] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Ky., Eastern Kentucky Hospital System and Cardiologist Agree to Collectively Pay More Than $3 Million to Resolve Civil Liability for Improper Healthcare Billings (Nov. 28, 2023), https://www.justice.gov/usao-edky/pr/eastern-kentucky-hospital-system-and-cardiologist-agree-collectively-pay-more-3.
[20] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, California Skilled Nursing Facilities, Owner and Management Company Agree to $45.6 Million Consent Judgement to Settle Allegations of Kickbacks to Referring Physicians (Nov. 15, 2023), https://www.justice.gov/opa/pr/california-skilled-nursing-facilities-owner-and-management-company-agree-456-million-consent.
[21] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, BioTelemetry and LifeWatch to Pay More than $14.7 Million to Resolve False Claims Act Allegations Relating to Remote Cardiac Monitoring Services (Dec. 18, 2023), https://www.justice.gov/opa/pr/biotelemetry-and-lifewatch-pay-more-147-million-resolve-false-claims-act-allegations.
[22] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Indiana Health Network Agrees to Pay $345 Million to Settle Alleged False Claims Act Violations (Dec. 19, 2023), https://www.justice.gov/opa/pr/indiana-health-network-agrees-pay-345-million-settle-alleged-false-claims-act-violations.
[23] See Press Release, U.S. Atty’s Office for the Southern Dist. of Tex., United Memorial Medical Center to Pay $2M Plus Additional Payments for Allegedly Causing False Claims Related to Excessive Cost Outlier Payments and Double Billing for Covid-19 tests (Dec. 20, 2023), https://www.justice.gov/usao-sdtx/pr/united-memorial-medical-center-pay-2m-plus-additional-payments-allegedly-causing-false.
[24] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Pharmaceutical Company Ultragenyx Agrees to Pay $6 Million for Allegedly Paying Kickbacks to Induce Claims for Its Drug Crysvita (Dec. 21, 2023), https://www.justice.gov/opa/pr/pharmaceutical-company-ultragenyx-agrees-pay-6-million-allegedly-paying-kickbacks-induce.
[25] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Mo., United States Reaches $9.1 Million Civil Settlement with Total Access Urgent Care over False Claims Allegations (Dec. 21, 2023), https://www.justice.gov/usao-edmo/pr/united-states-reaches-91-million-civil-settlement-total-access-urgent-care-over-false.
[26] See Press Release, U.S. Atty’s Office for the Southern Dist. of Cal., Phillips Respironics Pays $2.4 Million for Allegedly Giving Kickbacks (Dec. 22, 2023), https://www.justice.gov/usao-sdca/pr/phillips-respironics-pays-24-million-allegedly-giving-kickbacks.
[27] See Press Release, U.S. Atty’s Office for the Dist. of D.C., Booz Allen Agrees to Pay $377.45 Million to Settle False Claims Act Allegations (July 21, 2023), https://www.justice.gov/usao-dc/pr/booz-allen-agrees-pay-37745-million-settle-false-claims-act-allegations.
[28] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Mich., Federal Contractor Agrees to Pay $7 Million to Settle False Claims Act Allegations (July 21, 2023), https://www.justice.gov/usao-edmi/pr/federal-contractor-agrees-pay-7-million-settle-false-claims-act-allegations.
[29] See Press Release, U.S. Atty’s Office for the Northern Dist. of N.Y., Amphenol Corporation Pays $18 Million To Resolve Allegations That It Submitted False Claims for Electrical Connectors (Aug. 4, 2023), https://www.justice.gov/usao-ndny/pr/amphenol-corporation-pays-18-million-resolve-allegations-it-submitted-false-claims#_ftn1.
[30] See Press Release, U.S. Dep’t of Justice, Office of Pub. Affairs, Cooperating Federal Contractor Resolves Liability for Alleged False Claims Caused by Failure to Fully Implement Cybersecurity Controls (Sept. 5, 2023),
https://www.justice.gov/opa/pr/cooperating-federal-contractor-resolves-liability-alleged-false-claims-caused-failure-fully; Settlement Agreement, https://www.justice.gov/opa/file/1313011/dl?inline.
[31] See Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Navmar Applied Sciences Corporation Agrees to Pay $4.4 Million to Resolve Claims of Double-Billing and Cost-Shifting Under U.S. Navy Contracts (Sept. 15, 2023), https://www.justice.gov/usao-edpa/pr/navmar-applied-sciences-corporation-agrees-pay-44-million-resolve-claims-double.
[32] See, Press Release, U.S. Atty’s Office for the Eastern Dist. of Pa., Boeing to Pay $8.1 Million to Resolve Alleged False Claims Act Violations Arising from Manufacture of V-22 Osprey Aircraft (Sept. 28, 2023), https://www.justice.gov/usao-edpa/pr/boeing-pay-81-million-resolve-alleged-false-claims-act-violations-arising-manufacture.
[33] See Press Release, U.S. Atty’s Office for the Southern Dist. of Ohio, Virginia Tactical Gear & Equipment Company Agrees to Pay More than $2 Million to Settle Allegations Related to Buy American Act (Nov. 20, 2023), https://www.justice.gov/usao-sdoh/pr/virginia-tactical-gear-equipment-company-agrees-pay-more-2-million-settle-allegations.
[34] See Press Release, U.S. Atty’s Office for the Northern Dist. of Fl., Florida Real Estate Broker Agrees to Pay over $4 Million to Resolve False Claims Act Allegations Relating to Fraudulent Cares Act Loans (Aug. 16, 2023), https://www.justice.gov/usao-ndfl/pr/florida-real-estate-broker-agrees-pay-over-4-million-resolve-false-claims-act.
[35] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Victory Automotive Group Inc. Agrees to Pay $9 Million to Settle False Claims Act Allegations Relating to Paycheck Protection Program Loan (Oct. 11, 2023), https://www.justice.gov/opa/pr/victory-automotive-group-inc-agrees-pay-9-million-settle-false-claims-act-allegations.
[36] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, http://tinyurl.com/FCA-settlement.
[37] See Press Release, U.S. Atty’s Office for the Northern Dist. of N.Y., United States Attorney Freedman Announces First-Ever Settlement of False Claims Act Whistleblower Case Involving Grants for Restaurants and Similar Businesses Struggling During the COVID-19 Pandemic (Nov. 1, 2023), https://www.justice.gov/usao-ndny/pr/united-states-attorney-freedman-announces-first-ever-settlement-false-claims-act.
[38] See Press Release, U.S. Atty’s Office for the Northern Dist. of Tex., Dallas Importer and Two Chinese Companies to Pay $2.5 Million to Resolve Allegations of Underpaying Customs Duties (Dec. 5, 2023), https://www.justice.gov/usao-ndtx/pr/dallas-importer-and-two-chinese-companies-pay-25-million-resolve-allegations.
[39] See Press Release, U.S. Atty’s Office for the Dist. of N.J., Bergen County Public Relations Company Settles Allegations It Received Improper Paycheck Protection Program Loan (Dec. 7, 2023), https://www.justice.gov/usao-nj/pr/bergen-county-public-relations-company-settles-allegations-it-received-improper-paycheck.
[40] See Press Release, U.S. Atty’s Office for the Northern Dist. of Tex., National Roofing Company Settles PPP Fraud Allegations for $9 Million (Dec. 11, 2023), https://www.justice.gov/usao-ndtx/pr/national-roofing-company-settles-ppp-fraud-allegations-9-million.
[41] Jason Marcus, Bracker & Marcus Ties for the Largest PPP Settlement on Record (last visited Feb. 10, 2023), https://www.fcacounsel.com/blog/bracker-marcus-ties-for-the-largest-ppp-settlement-on-record/.
[42] Justice Manual 4-4.112, Guidelines for Taking Disclosure, Cooperation, and Remediation into Account in False Claims Act Matters (May 2019), https://www.justice.gov/jm/jm-4-4000-commercial-litigation#4-4.112.
[43] Id.
[44]Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Department of Justice Issues Guidance on False Claims Act Matters and Updates Justice Manual (May 7, 2019), https://www.justice.gov/opa/pr/department-justice-issues-guidance-false-claims-act-matters-and-updates-justice-manual.
[45] See, e.g., Speech, U.S. Dep’t of Justice, Deputy Attorney General Lisa Monaco Delivers Remarks at American Bar Association National Institute on White Collar Crime (Mar. 2, 2023), https://www.justice.gov/opa/speech/deputy-attorney-general-lisa-monaco-delivers-remarks-american-bar-association-national.
[46]U.S. Dep’t of Justice, U.S. Attorneys’ Offices Voluntary Self-Disclosure Policy (Feb. 22, 2023); see, e.g., https://www.justice.gov/d9/pages/attachments/2023/02/23/usao_voluntary_self-disclosure_policy.pdf; https://www.justice.gov/criminal-fraud/file/1562831/dl; https://www.justice.gov/d9/2023-04/NSD%20VSD%20Policy%20-3.1.23.pdf.
[47]Id. See https://www.justice.gov/corporate-crime/voluntary-self-disclosure-and-monitor-selection-policies.
[48]Speech, Office of Pub. Affairs, U.S. Dep’t of Justice, Deputy Attorney General Lisa O. Monaco Announces New Safe Harbor Policy for Voluntary Self-Disclosures Made in Connection with Mergers and Acquisitions (Oct. 4, 2023), https://www.justice.gov/opa/speech/deputy-attorney-general-lisa-o-monaco-announces-new-safe-harbor-policy-voluntary-self.
[49]Deferred Prosecution Agreement, United States v. Freepoint Commodities, LLC, No. 3-23-cr-224-KAD (Dec. 12, 2023) https://www.justice.gov/opa/media/1329266/dl?inline, at ¶ 4.
[50] Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Cooperating Federal Contractor Resolves Liability for Alleged False Claims Caused by Failure to Fully Implement Cybersecurity Controls (Sept. 5, 2023), https://www.justice.gov/opa/pr/cooperating-federal-contractor-resolves-liability-alleged-false-claims-caused-failure-fully.
[51] Id.
[52] Settlement Agreement, U.S. Dep’t of Justice and Verizon Business Network Services LLC (Sept. 5, 2023), https://www.justice.gov/opa/file/1313011/dl?inline.
[53] Press Release, U.S. Atty’s Office for Eastern Dist. of Ky., Eastern Kentucky Hospital System and Cardiologist Agree to Collectively Pay More Than $3 Million to Resolve Civil Liability for Improper Healthcare Billings (Nov. 28, 2023), https://www.justice.gov/usao-edky/pr/eastern-kentucky-hospital-system-and-cardiologist-agree-collectively-pay-more-3.
[54] Settlement Agreement, U.S. Dep’t of Justice and VitalAxis, Inc. (June 15, 2023), https://www.justice.gov/d9/press-releases/attachments/2023/06/16/settlement_agreement_-_vitalaxis_-_signed_redacted.pdf.
[55] Settlement Agreement, U.S. Dep’t of Justice and MWW Group LLC (Oct. 27, 2023), https://www.justice.gov/usao-nj/media/1327611/dl?inline.
[56] Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Deputy Attorney General Lisa O. Monaco Announces New Civil Cyber-Fraud Initiative (Oct. 6, 2021), https://www.justice.gov/opa/pr/deputy-attorney-general-lisa-o-monaco-announces-new-civil-cyber-fraud-initiative?utm_medium=email&utm_source=govdelivery.
[57] Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Cooperating Federal Contractor Resolves Liability for Alleged False Claims Caused by Failure to Fully Implement Cybersecurity Controls (Sept. 5, 2023), https://www.justice.gov/opa/pr/cooperating-federal-contractor-resolves-liability-alleged-false-claims-caused-failure-fully.
[58] See United States ex rel. Matthew Decker v. Pennsylvania State University, 22-cv-03895-PD (E.D. Pa. Oct. 5, 2022).
[59]See U.S. Dep’t of Defense, Gen. Servs. Admin., and Nat’l Aeronautics and Space Admin., Federal Acquisition Regulation: Cyber Threat and Incident Reporting and Information Sharing (FAR Case 2021-017) (Oct. 3, 2023), https://www.federalregister.gov/documents/2023/10/03/2023-21328/federal-acquisition-regulation-cyber-threat-and-incident-reporting-and-information-sharing.
[60] See Office of Inspector General, U.S. Dep’t of Health & Hum. Servs., General Compliance Program Guidance (Nov. 2023), https://oig.hhs.gov/compliance/general-compliance-program-guidance/.
[61] Id. at 18.
[62] Id. at 19.
[63] See id. at 32.
[64] Id. at 58.
[65] Id. at 61.
[66] Id.
[67] See 42 U.S.C. § 1320a-7k.
[68] See General Compliance Program Guidance at 7.
[69] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Justice Department Announces Results of Nationwide COVID-19 Fraud Enforcement Action (Aug. 23, 2023), https://www.justice.gov/opa/pr/justice-department-announces-results-nationwide-covid-19-fraud-enforcement-action.
[70] See id.
[71] S.B. 426, 2022 Gen. Assemb. (Conn. 2022); Pub. Act 23-129—HB 6826, Office of Legislative Research Public Act Summary (https://www.cga.ct.gov/2023/SUM/PDF/2023SUM00129-R02HB-06826-SUM.PDF).
[72] See Senate Budget and Appropriations Committee Statement to Senate Bill No. 4018, State of New Jersey, June 27, 2023 (https://pub.njleg.state.nj.us/Bills/2022/S4500/4018_S1.HTM); State False Claims Act Reviews, U.S. Dep’t of Health and Human Servs, Office of Inspector General, last visited Jan. 19, 2024 (https://oig.hhs.gov/fraud/state-false-claims-act-reviews/).
[73] State False Claims Act Reviews, U.S. Dep’t of Health and Human Servs, Office of Inspector General, https://oig.hhs.gov/fraud/state-false-claims-act-reviews/ (last visited Jan. 19, 2024).
[74] Id.
[75] Senate Budget and Appropriations Committee Statement to Senate Bill No. 4018, State of New Jersey, June 27, 2023 (https://pub.njleg.state.nj.us/Bills/2022/S4500/4018_S1.HTM).
[76] 2023 Legislative Agenda on Public Health, Washington State, Office of the Attorney General, https://www.atg.wa.gov/2023-legislative-agenda (last visited Jan. 19, 2023).
[77] 2023 AG Request Legislation, Protecting Whistleblower Provision of the Medicaid False Claims Act, Attorney General of Washington, 2023 (https://agportal-s3bucket.s3.amazonaws.com/uploadedfiles/Another/Office_Initiatives/Medicaid%20FCA%20Whistleblower.pdf).
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues and are available to assist in addressing any questions you may have regarding these issues. Please contact the Gibson Dunn lawyer with whom you usually work, the authors, or any leader or member of the firm’s False Claims Act/Qui Tam Defense practice group:
Washington, D.C.
Jonathan M. Phillips – Co-Chair (+1 202.887.3546, jphillips@gibsondunn.com)
F. Joseph Warin (+1 202.887.3609, fwarin@gibsondunn.com)
Gustav W. Eyler (+1 202.955.8610, geyler@gibsondunn.com)
Lindsay M. Paulin (+1 202.887.3701, lpaulin@gibsondunn.com)
Geoffrey M. Sigler (+1 202.887.3752, gsigler@gibsondunn.com)
Joseph D. West (+1 202.955.8658, jwest@gibsondunn.com)
San Francisco
Winston Y. Chan – Co-Chair (+1 415.393.8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415.393.8391, cstevens@gibsondunn.com)
New York
Reed Brodsky (+1 212.351.5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212.351.3850, mdenerstein@gibsondunn.com)
Alexander H. Southwell (+1 212.351.3981, asouthwell@gibsondunn.com)
Denver
Ryan T. Bergsieker (+1 303.298.5774, rbergsieker@gibsondunn.com)
Robert C. Blume (+1 303.298.5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303.298.5784, mloseman@gibsondunn.com)
John D.W. Partridge (+1 303.298.5931, jpartridge@gibsondunn.com)
Dallas
Andrew LeGrand (+1 214.698.3405, alegrand@gibsondunn.com)
Los Angeles
Nicola T. Hanna (+1 213.229.7269, nhanna@gibsondunn.com)
Jeremy S. Smith (+1 213.229.7973, jssmith@gibsondunn.com)
Deborah L. Stein (+1 213.229.7164, dstein@gibsondunn.com)
James L. Zelenay Jr. (+1 213.229.7449, jzelenay@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650.849.5395, bwagner@gibsondunn.com)
*Nicole Waddick is an associate practicing in the firm’s San Francisco office who currently is not admitted to practice law.
© 2024 Gibson, Dunn & Crutcher LLP. All rights reserved. For contact and other information, please visit us at www.gibsondunn.com.
Attorney Advertising: These materials were prepared for general informational purposes only based on information available at the time of publication and are not intended as, do not constitute, and should not be relied upon as, legal advice or a legal opinion on any specific facts or circumstances. Gibson Dunn (and its affiliates, attorneys, and employees) shall not have any liability in connection with any use of these materials. The sharing of these materials does not establish an attorney-client relationship with the recipient and should not be relied upon as an alternative for advice from qualified counsel. Please note that facts and circumstances may vary, and prior results do not guarantee a similar outcome.
A dull year is rare when it comes to the False Claims Act (FCA), but this last year was exceptional by any standard. In the last twelve months, the Supreme Court decided to take up two different issues under the FCA, while the Department of Justice (DOJ) announced, yet again, billions in recoveries and nearly a thousand new FCA cases, a new record.
DOJ’s $2.2 billion in recoveries during FY 2022 marked the fourteenth straight year where recoveries exceeded $2 billion, dating back to 2008. But even more notable than the dollar amount was the sheer volume of FCA activity. DOJ obtained its recoveries from the second-highest number of settlements in history, and there were more new FCA matters initiated in FY 2022 than in any prior year, meaning the pipeline of FCA lawsuits is very full.
As in past years, FCA recoveries in the healthcare and life sciences industries continued to dominate enforcement activity in terms of the number and value of settlements, including several seven- and eight-figure settlements for alleged kickback schemes during the second half of the year. Meanwhile, notwithstanding the relatively few FCA enforcement actions related to COVID-19 in 2022, the government also signaled that it continues to take pandemic-related fraud seriously, and we expect to see increasing FCA enforcement in response to conduct arising out of the pandemic.
If there was one quiet area this year, it was on the legislative front. The FCA Amendments Act of 2021—which briefly gained momentum last year as Senator Chuck Grassley (R-IA) pushed to overhaul the FCA—remained at a standstill in Congress, and there were no other major developments in FCA legislation (federal or state).
But activity in the courts more than made up for the lack of legislation. As noted above, the Supreme Court took up two critical issues under the FCA. In December, the Supreme Court heard argument about the level of scrutiny that applies when DOJ seeks to dismiss an FCA case over the whistleblower’s objection. And just last month, the Supreme Court agreed to hear a case concerning the scienter standard under the FCA, which will have important implications for the scope of FCA liability in cases premised on alleged statutory or regulatory violations in ambiguous areas of law. Meanwhile, federal circuit courts also continued to consider the FCA’s pleading standards under Rule 9(b); the relationship between the anti-kickback statute and the FCA; and the FCA’s materiality standard, among other FCA issues.
* * *
We cover all of this, and more, below. We begin by summarizing recent enforcement activity, then provide an overview of notable legislative and policy developments at the federal and state levels, and finally analyze significant court decisions from the past six months.
As always, Gibson Dunn’s recent publications regarding the FCA may be found on our website, including in-depth discussions of the FCA’s framework and operation, industry-specific presentations, and practical guidance to help companies avoid or limit liability under the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
I. FCA ENFORCEMENT ACTIVITY
A. NEW FCA ACTIVITY
There were more new FCA cases filed in 2022 than in any year in history.[1] The government and qui tam relators filed 948 new cases, surpassing the previous record set in 2020, when there were 922 new cases. This new high-water mark shows that the volume of FCA activity is only accelerating.
Of the new cases, the government itself initiated 296 cases (referrals and investigations) outside of the qui tam setting, which is also a new record, both in terms of the total number of FCA cases initiated by the government, and as a percentage of the total number of new FCA cases.[2] In other words, the Department of Justice is bringing FCA cases on its own accord at an unprecedented pace.
This is extremely important because, historically, the vast majority of FCA recoveries come in cases where the government either brings the case or later intervenes. The relatively high level of activity from the government, therefore, suggests that recoveries in future years could be on the rise.
Number of FCA New Matters, Including Qui Tam Actions
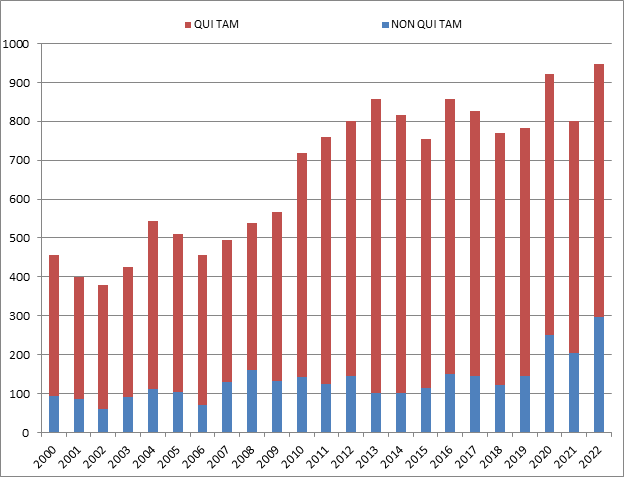
Source: DOJ “Fraud Statistics – Overview” (Feb. 7, 2023)
B. TOTAL RECOVERY AMOUNTS: 2022 RECOVERIES EXCEED $2.2 BILLION
While most metrics point to a banner year, the total dollars recovered in FY 2022 ($2.2 billion) was down considerably from 2021 (when DOJ announced more than $5.6 billion), and the lowest more than a decade. This appears to reflect a relatively low number of blockbuster settlements (e.g., those in the nine-figure range). Nonetheless, it was the fourteenth consecutive year, dating back to 2008, that DOJ announced more than $2 billion in recoveries.[3]
Although the dollar value of recoveries is the lowest in more than a decade, the FCA enforcement activity is as high as ever. Indeed, DOJ touted that “[t]he government and whistleblowers were party to 351 settlements and judgments, the second-highest number of settlements and judgments in a single year.”[4] In other words, even if the dollar figures were not record setting, the number of successful DOJ cases was.
Whistleblower activity also remains a critical part of the FCA. Of the $2.2 billion in recoveries DOJ reported, more than $1.9 billion came from lawsuits that were initially filed under the qui tam provisions of the FCA (and then pursued by either the government or whistleblowers).[5] This is consistent with historical trends.
A more unusual datapoint this year was the percentage obtained in cases where the U.S. declined to intervene. Historically, DOJ’s decision on whether to intervene is a critical inflection point that strongly predicts whether a case will be successful: this makes sense, as DOJ is more likely to intervene in cases that it believes are “winners.” But this year, a remarkable 54% of recoveries were in non-intervened cases. This number is strongly skewed, however, by a single case against a pharmaceutical company where DOJ did not intervene and the Relator obtained a settlement of nearly $900 million. If that case is removed, then the data looks much more consistent with historical trends—suggesting the continued importance of DOJ intervention decisions.
Settlements or Judgments in Cases Where the Government Declined Intervention as a Percentage of Total FCA Recoveries
Source: DOJ “Fraud Statistics – Overview” (Feb. 7, 2023)
C. FCA Recoveries by Industry
The relative breakdown of FCA recoveries across industries remained relatively consistent with past years. Healthcare cases comprised 80% of total recoveries, Department of Defense procurement issues made up 5%, and the remaining 15% was split among other industries.[6]
Within the healthcare industry, DOJ announced significant recoveries across a range of theories, including Medicaid fraud, unnecessary and substandard care, kickbacks, and Medicare Advantage fraud. DOJ also announced significant recoveries from Department of Defense contractors, and, as discussed further below, the beginnings of significant COVID-19 related activity.[7]
FCA Recoveries by Industry
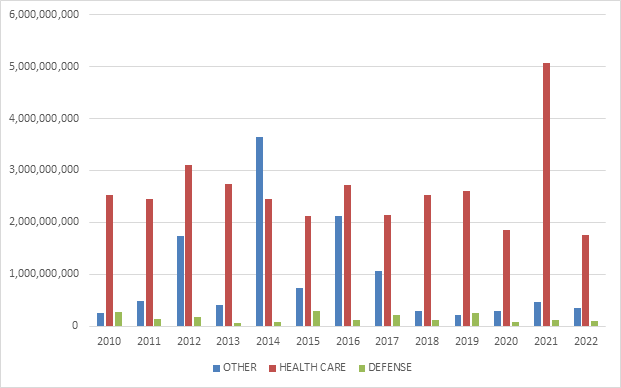
Source: DOJ “Fraud Statistics – Overview” (Feb. 7, 2023)
II. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE SECOND HALF OF 2022
We summarize below the most notable FCA settlements in the second half of calendar year 2022, with a focus on the industries and theories of liability involved. We covered settlements from the first half of the year in our 2022 Mid-Year Update.
FCA recoveries in the healthcare and life sciences industries continued to dominate enforcement activity during the second half of the year in terms of the number and value of settlements.
- On June 28, 15 Texas-based doctors agreed to pay a total of $2.83 million to settle allegations that they violated the FCA by accepting illegal kickbacks in exchange for patient referrals to three companies providing laboratory testing services. The government alleged that one of the testing companies paid “volume-based commissions” to independent recruiters, who, in turn, used management service organizations (MSOs) to pay the doctors for their referrals to the testing companies. The payments from the MSOs to the doctors “were allegedly disguised as investment returns but in fact were based on, and offered in exchange for, the doctors’ referrals.” As of June 28, the United States had reached settlements with 33 physicians, two executives, and a laboratory in the same alleged scheme, and in May 2022, it filed a FCA lawsuit in the Eastern District of Texas against, inter alia, the three chief executive officers of the testing companies. See United States ex rel. STF, LLC v. True Health Diagnostics, LLC, No. 4:16-cv-547 (E.D. Tex.).[8]
- On June 30, a Florida nursing and assisted living system agreed to pay $1.75 million to resolve allegations that it violated the FCA by providing COVID-19 vaccinations for hundreds of ineligible individuals. The government alleged that the system invited and facilitated vaccines for board members, donors and potential donors, and other ineligible individuals as part of the Centers for Disease Control and Prevention’s (CDC) Pharmacy Partnership for Long-Term Care Program, which was designed to vaccinate long-term care facility residents and staff when doses of COVID-19 vaccine were in limited supply early in the CDC vaccination program.[9]
- On July 1, a spinal implant devices distributor headquartered in Utah, its two owners, and two of their physician-owned distributorships agreed to pay $1 million to resolve a lawsuit against them alleging that they violated the FCA by paying purported kickbacks to physicians. The government alleged that the distributor’s physician-owned distributorships allowed them to pay physicians to use the distributor’s medical devices in surgeries. The settlement was reached after the first day of trial.[10]
- On July 7, a West Virginia hospital agreed to pay $1.5 million to resolve allegations that it violated the FCA by knowingly submitting or causing the submission of claims to Medicare in violation of the Stark Law. The settlement stems from the hospital’s voluntary self-disclosure of potential violations of the Stark Law by paying compensation to referring physicians that allegedly exceeded fair market value or took into account the volume or value of the physicians’ referrals to the hospital.[11]
- On July 13, a Florida-based pharmacy entered into a deferred prosecution agreement (DPA) and agreed to pay a $1.31 million civil settlement to resolve allegations that it submitted fraudulent claims to Medicare for a high-priced drug used in rapid reversal of opioid overdoses. The government alleged that the pharmacy completed prior authorization forms for the drug in place of the prescribing physicians, including instances in which the pharmacy staff signed the forms without the physician’s authorization and listed the pharmacy’s contact information as if it were the physician’s information. The government further alleged that the pharmacy submitted prior authorization requests for the drug that contained false clinical information to secure approval for the expensive drug. In connection with the settlement, HHS agreed to release its right to exclude the pharmacy and its CEO in exchange for their agreements to enter into a three-year Integrity Agreement with the U.S. Department of Health, Office of Inspector General (HHS OIG) that requires, among other things, the pharmacy to implement measures designed to ensure that its submission of claims for pharmaceutical products complies with applicable law relating to prior authorizations and collection of beneficiary co-payment obligations. The settlement resolves claims brought in a qui tam suit by a former employee of the manufacturer of the drug. As part of this resolution, the relator will receive $262,000 of the settlement amount.[12]
- On July 14, a New Jersey company providing laboratory testing services and its corporate parent agreed to pay $9.85 million to resolve alleged violations of the FCA arising from the company’s payment of above-market rents to physician landlords for office space in exchange for patient referrals from those physicians. The testing company rented office space from several physicians and physicians’ groups for Patient Service Centers (PSCs), where it collected blood samples from patients. In the settlement agreement, the testing company admitted that it artificially inflated its rental payments to physician landlords for the PSCs by (i) “inaccurately measur[ing] the amount of space that [it] would use exclusively,” and (ii) “includ[ing] a disproportionate share of the common spaces” in the calculations of office space for which it made payments. The testing company also admitted that it considered the number of referrals it received from physician landlords “when deciding whether to open, maintain or close” the PSCs, and that both the testing company and its parent entity had conducted internal audits that previously identified some of the above-market lease payments but did not report these findings to the Federal Government. Under the settlement, the testing company and its parent entity will also pay the Commonwealth of Massachusetts and the State of Connecticut $141,041 and $5,001, respectively, to resolve alleged violations of those states’ FCA statutes, and the testing company entered into a separate Corporate Integrity Agreement (CIA) with HHS OIG. The settlement also resolved claims brought under a qui tam suit filed by a former employee of both the testing company and the parent entity; the whistleblower will receive $1.7 million as her share of the recovery.[13]
- On July 20, a Texas-headquartered clinical laboratory agreed to pay $16 million to resolve allegations that it submitted false claims for payment to federal healthcare programs, including Medicare. The government alleged that the clinical laboratory systematically conducted unnecessary additional testing on biopsy specimens prior to a pathologist’s review and without an individualized determination confirming the legitimate necessity for additional testing. The settlement resolved a qui tam suit filed by a relator who received $2.72 million of the recovery amount.[14]
- On July 22, an Oregon-based medical device manufacturer agreed to pay $12.95 million to settle allegations that it violated the FCA by paying illegal kickbacks to physicians to induce their use of the manufacturer’s pacemakers, defibrillators, and other implantable cardiac devices. The settlement resolved allegations that the manufacturer made excessive payments to physicians it hired to train its new employees, such as “for training events that either never occurred or were of little or no value to the trainees,” to induce or reward the physicians’ use of the manufacturer’s devices. Additionally, the settlement resolved separate allegations that the manufacturer paid physicians illegal kickbacks in the form of “holiday parties, winery tours, lavish meals with no legitimate business purpose and international business class airfare and honoraria in exchange for making brief appearances at international conferences.” The settlement also includes a resolution of claims brought in a qui tam suit by two of the manufacturer’s former sales representatives, who will receive approximately $2.1 million total as their share of the recovery.[15]
- On July 22, clinical laboratories in Mississippi and Texas and two of their owner/operators agreed to pay $5.7 million to resolve allegations that they caused the submission of false claims to Medicare by paying kickbacks in return for genetic testing samples. The government alleged that the laboratories and their owner/operators participated in a genetic testing fraud scheme with various marketers whereby the marketers solicited genetic testing samples from Medicare beneficiaries and arranged to have a physician fraudulently attest that the genetic testing was medically necessary. The laboratories would then process the tests, receive reimbursement from Medicare, and pay a portion of that reimbursement to the marketers. The owner/operators have each previously pled guilty to one count of conspiracy to defraud the United States in connection with this scheme.[16]
- On August 18, a California organized health system and three medical care providers agreed to pay a total of $70.7 million to settle allegations that they broke federal and state laws by submitting or causing the submission of false claims to Medi-Cal related to Medicaid Adult Expansion under the Patient Protection and Affordable Care Act. The settlements resolve allegations that the parties knowingly submitted or caused the submission of false claims to Medi-Cal for “Additional Services” provided to Adult Expansion Medi-Cal members between January 1, 2014 and May 31, 2015. In addition to the FCA settlement, two of the parties entered into a CIA. The settlements resolve claims brought by qui tam relators under the FCA and the California False Claims Act.[17]
- On August 23, a Texas company that manufactures, markets, and distributes optical lenses agreed to pay $16.4 million to resolve allegations that it violated the FCA by paying illegal kickbacks to eye care providers, such as optometrists and ophthalmologists, to induce them to prescribe the company’s products to patients. The company also entered into a CIA. The settlement also resolved claims brought in two qui tam suits filed by several of the company’s former district sales managers; the whistleblowers’ share of the recovery was not reported.[18]
- On September 1, a manufacturer of durable medical equipment (DME) agreed to pay $24 million to settle allegations that it misled multiple federal healthcare programs by paying kickbacks to DME suppliers. The government alleged that the manufacturer cased DME suppliers to submit false claims for oxygen concentrators, ventilators, CPAP and BiPAP machines, and other respiratory-related medical equipment because the manufacturer provided illegal inducements to the DME suppliers by providing them with physician prescribing data free of charge that could assist their marketing efforts. The settlement required the manufacturer to pay $22.62 million to the United States, and $2.13 million to various states as a result of the impact to their Medicaid programs, pursuant to the terms of separate settlement agreements entered into with the respective states. Additionally, the manufacturer entered into a CIA. The settlement also resolved a qui tam lawsuit brought by an employee of the manufacturer, who received approximately $4.3 million of the recovery amount.[19]
- On September 2, a pharmaceutical manufacturer agreed to pay $40 million to resolve allegations that the manufacturer paid kickbacks to hospitals and physicians to induce them to utilize certain drugs, marketed these drugs for off-label uses that were not reasonable and necessary, and downplayed the safety risks of a drug used to control bleeding in certain heart surgeries. The government also alleged that the manufacturer downplayed the efficacy and health risks associated with a drug used to treat cholesterol and induced a government agency to renew certain contracts relating to the same drug. The settlement resolved allegations brought in two qui tam suits by a former employee, who will receive approximately $11 million from the proceeds of the settlement.[20]
- On September 13, DOJ announced a settlement with a Texas bank for allegedly processing a Paycheck Protection Program (PPP) loan on behalf of an ineligible borrower. The U.S. Attorney’s Office for the Southern District of Texas, which brought the case, described the settlement as the “first-ever” settlement under the FCA from a PPP “lender”—i.e., the bank that made the loan, not a fraudulent borrower.[21]
- On September 15, a pharmaceutical company agreed to pay $7.9 million to settle allegations that it violated the FCA by causing the submission of claims to Medicare Part D for several generic drugs that utilized outdated “prescription-only” (“Rx-only”) labeling, even though the drugs had lost their Rx-only status and thus were no longer reimbursable under Medicare Part D. Under federal law, pharmaceuticals that require a prescription to be dispensed (i.e., Rx-only drugs) are reimbursable under Medicare Part D, whereas pharmaceuticals that do not require a prescription—and can be sold to customers over the counter (OTC)—are not eligible for reimbursement. As part of the settlement, the pharmaceutical company admitted that in order to boost profits, it delayed seeking conversion of three generic drugs it manufactures “even after learning that the brand-name drugs for each had converted to OTC status,” and that it continued to sell the drugs under “obsolete Rx-only labeling” rather than taking the drugs off the market. The pharmaceutical company received credit under the DOJ’s prosecution guidelines for disclosure, cooperation, and remediation. The settlement also resolved claims brought by a whistleblower in a qui tam suit; the whistleblower will receive approximately $946,000 as their share of the recovery.[22]
- On September 26, a pharmaceutical company agreed to pay $900 million to resolve allegations that it caused false claim submissions to Medicare and Medicaid by paying kickbacks to physicians as part of a scheme to induce them to prescribe the pharmaceutical company’s drugs. The allegations stem from a qui tam lawsuit filed by a former employer of the pharmaceutical company; the government declined to intervene in the lawsuit, which the whistleblower pursued individually. The whistleblower’s complaint alleged that the company offered and paid remuneration in various forms to induce physicians to prescribe the company’s drugs, including speaker honoraria and training fees, consulting fees to healthcare professionals who participated in the company’s speaker programs, training meetings, or consultant programs. The whistleblower received 29.6% or approximately $266 million from the settlement proceeds, the largest single whistleblower award on record, according to the whistleblower’s attorney. The $900 million settlement is also the largest recovery ever in a declined case.[23]
- On October 12, several pharmacy companies agreed to pay nearly $6.9 million to settle allegations that they violated the FCA by waiving patient copays, overcharging government health insurance programs, and trading healthcare business after they were removed from networks. Specifically, the government alleged that a compounding pharmacy and a related entity, created to handle the compounding pharmacy’s billing, created a copay-waiver program for patients and misled the government about the price being charged to uninsured, cash-paying patients by stating that that price was higher than it was, resulting in TRICARE beneficiaries being charged more than uninsured, cash-paying patients. The government also alleged that the compounding pharmacy sold its out-of-network prescriptions to other pharmacies after it was removed by some networks and received a portion of proceeds back. The settlement resolved allegations brought in a qui tam suit by a former accountant of the compounding pharmacy. She will receive approximately $1.4 million as her share of the recovery from the settlement.[24]
- On October 17, a healthcare services provider in California agreed to pay approximately $13 million to resolve allegations that it violated the FCA by billing federal insurance programs for urine toxicology tests that it did not actually perform. The United States alleged that under the terms of a contract with another company, urine toxicology specimens from physicians and laboratories were forwarded to the healthcare services provider. The United States contended that the provider sought reimbursement from the federal government for thousands of tests that it did not perform and that “were instead performed by third-party labs.”[25]
- On October 18, an Oklahoma-based for-profit home health provider, its affiliates, and the President and COO agreed to pay $7.2 million to resolve allegations that they violated the FCA by billing the Medicare program for medically unnecessary therapy provided to patients in Florida. The government alleged that the home health provider billed Medicare knowingly and improperly for home healthcare patients in Florida based on therapy provided without regard to medical necessity and overbilled for therapy by upcoding patients’ diagnoses. Both the President and COO agreed to be excluded from participation in all federal healthcare programs for a period of five years. The home health provider agreed to be bound by the terms of a CIA with HHS OIG. The settlement resolves a qui tam action brought by therapists formerly employed by the home health provider. The relators will together receive $1.3 million as their share of the settlement. Contemporaneous with the settlement, the home health provider agreed to pay an additional $22.9 million to resolve another qui tam action brought in the Western District of Oklahoma which alleged that the home health provider improperly paid remuneration to its home health medical directors in Oklahoma and Texas for the purpose of inducing referrals of Medicare and TRICARE home health patients.[26]
- On November 1, a cloud-based electronic health record (EHR) technology vendor agreed to pay $45 million to settle allegations that it violated the FCA by accepting and paying unlawful remuneration in exchange for referrals through multiple kickback schemes. The government alleged that the EHR technology vendor, who sells EHR systems subscriptions services, petitioned and accepted kickbacks in exchange for recommending and arranging for its users to utilize another company’s pathology laboratory services. Additionally, the EHR technology vendor allegedly conspired with the same third-party laboratory to improperly donate EHR to healthcare providers with the goal to increase lab orders for the third-party laboratory and concurrently increase the EHR technology vendor’s user base. The government further alleged that the EHR technology vendor paid kickbacks to its customers and to other influential parties in the healthcare industry to secure recommendations and referral for its EHR The settlement also resolved, in part, the qui tam lawsuit filed by a former vice president of the EHR technology vendor, who received approximately $9 million from the settlement agreement.[27]
- On November 9, the successor in interest to a Tennessee-based real estate investment trust agreed to pay $3 million to resolve allegations that the trust violated the FCA by submitting false claims to Medicare and Medicaid. The government alleged that the trust paid kickbacks to physicians to induce them to refer patients to a hospital developed by an affiliated party. The government alleged that the trust offered the physicians a low-risk, high-reward investment in a joint venture formed by one of the parties to purchase the hospital and lease it back to the affiliated party. The allegations were initially brought in a qui tam lawsuit by two relators. The qui tam suit remains under seal, subject to an order of the Court permitting the United States to disclose the settlement.[28]
- On November 10, a birth-related injury compensation plan created by the State of Florida and the plan’s administrator agreed to pay $51 million to settle a qui tam lawsuit alleging that it violated the FCA by causing participants in the plan to submit covered claims to Medicaid rather than to the compensation plan, contrary to “Medicaid’s status as the payer of last resort under federal law.” The whistleblowers will receive $12,750,000 as their share of the recovery. While the United States did not intervene in the case, it assisted the whistleblowers with defending against a motion to dismiss filed by the defendants and with negotiating the settlement.[29]
- On December 5, a New Jersey based opioid abuse treatment facility agreed to pay $3.15 million to settle civil and criminal allegations that it paid kickbacks, obstructed a federal audit, and submitted fraudulent claims to Medicaid. The government alleged that the facility submitted false claims to Medicaid related to a kickback relationship with a methadone mixing company with whom it shared a related ownership and management history. The settlement further resolved allegation that the facility failed to maintain adequate supervision and staffing, relying instead on non-credentialed interns to provide services. Related to the criminal allegations, the facility agreed to enter into a three-year deferred prosecution agreement that requires it to abide by certain measures, including, among other things, creating an independent board of advisors to oversee the company’s compliance relating to federal healthcare laws.[30]
- On December 12, a not-for-profit health system, community hospital, and medical center agreed to pay $22.5 million to resolve allegations that they violated the FCA and California FCA by submitting claims for services that were unallowable medical expenses under the contract between the California’s Department of Health Care Services and a California county organized health system. The settlement also resolved allegations that the reimbursements for services did not reflect the fair market value of services provided and that services were duplicative of services already required to be rendered. Further, the government alleged that the payments were unlawful gifts of public funds in violation of the California Constitution. The settlement also resolved allegations brought in a qui tam suit by a former medical director of a California county organized health system, who will received $3.9 million as his share of the federal recovery.[31]
- On December 13, a Jacksonville-based company and its subsidiary agreed to pay $3 million to resolve allegations that they violated the FCA by paying and receiving kickbacks in connection with genetic testing samples. The government alleged that the companies solicited genetic testing samples from Medicare beneficiaries and paid physicians to falsely attest that the genetic testing was medically necessary and arranged for laboratories to process the tests. The laboratories would pay a portion of the reimbursement to the company. The settlement also resolved allegations brought in a qui tam suit by two individuals who were approached to participate in the scheme. They received approximately $570,000 as their share of the recovery.[32]
III. LEGISLATIVE AND POLICY DEVELOPMENTS
A. FEDERAL POLICY AND LEGISLATIVE DEVELOPMENTS
1. Changes to Rules Regarding Overpayments
On December 14, CMS issued a proposed rule which would, among other things, change the standard for what it means for Medicare program participants to “identify” overpayments.[33] The Affordable Care Act requires any person who has received an overpayment from a federal healthcare program to report and return that overpayment within 60 days after it is “identified.” Under Medicare rules issued in 2014 and 2015, CMS advised that a program participant has “identified” an overpayment when it “has, or should have through the exercise of reasonable diligence, determined” that it received an overpayment.[34] The definition is significant to the FCA because, once an overpayment is “identified,” then an “obligation” may exist under the “reverse false claim” provision of the FCA, which prohibit acts of fraud aimed at avoiding paying money to the United States, if the overpayment is not returned within the required 60-day period.[35]
CMS’s stated rationale behind its interpretation of the term “identified” was that it would align with the FCA’s knowledge requirement, which creates fraud liability if an overpayment is improperly retained with actual knowledge, reckless regard, or deliberate ignorance of that overpayment. In 2018, however, a federal district court ruled that to the contrary, the “should have through the exercise of reasonable diligence” standard created by CMS has the effect of punishing simple negligence instead.[36] In direct response to that opinion, the new proposed rule would eliminate the “reasonable diligence” standard and instead deem an overpayment “identified” when a program participant—consistent with the scienter requirement of the FCA—has actual knowledge of an overpayment, or deliberately ignores or recklessly disregards an overpayment.[37]
In a related development, CMS finalized a rule on January 30, 2023 that enhances the government’s audit powers over Medicare Advantage plans (i.e., Medicare “private” plans).[38] The rule provides that, when seeking to collect overpayments from Medicare Advantage plans via Risk Adjustment Data Validation (RADV) audits, CMS will extrapolate audit findings for the relevant year forward to all payment years; however it will do so starting only with payment year (PY) 2018.[39] This is an important change from the initial proposed rule, which would have called for extrapolation starting in PY 2011.[40] The new RADV rule nevertheless has the potential to significantly expand the universe of risk adjustment data that is subject to audit, as the RADV audits are a primary program integrity tool for CMS in overseeing the Medicare Advantage program. That, in turn, creates additional potential “obligations” under the FCA and the forthcoming new CMS interpretative guidance regarding the 60-Day Rule, as applied to Medicare Advantage plans. Medicare Advantage plans have disputed many other aspects of this audit process and proposed rule, and we anticipate that those disputes will continue to play out in various contexts, including several ongoing FCA cases on related topics and issues. We will be tracking further developments stemming from the new rule as 2023 unfolds.
2. Enforcement Efforts Related to COVID‑19
Based on publicly available settlements, civil FCA enforcement actions related to COVID‑19 spending have been relatively few in number in relation to the Justice Department’s criminal enforcement activity. Indeed, if one were to compare sheer public displays of enforcement activity and resource commitment in the criminal versus civil realms, it would be easy to wonder whether civil enforcement is lagging behind criminal prosecutions. In September, for example, DOJ announced the establishment of three Strike Force teams, which will operate out of U.S. Attorney’s Offices in the Southern District of Florida, in the District of Maryland, and in California as a joint effort between the Central and Eastern Districts of California.[41] The prosecutor-driven Strike Force teams will “accelerate the process of turning data analytics into criminal investigations,” according to DOJ.[42]
As discussed in our 2021 Year-End Update, however, early civil enforcement activity is likely only the start of a years-long effort by DOJ to wield the FCA to combat fraud related to pandemic relief funds. Because FCA cases are filed under seal, and often take years to investigate, we may not see the full extent of pandemic-related FCA activity for years to come. But developments in the second half of 2022 lend support to the idea that DOJ is playing a long game when it comes to civil enforcement in areas affected by the pandemic.
We see this in part in developments at HHS OIG, one of DOJ’s most frequent partner agencies in FCA enforcement. In September, HHS OIG released the results of a study into telehealth services provided during the first year of the pandemic, including a description of “providers’ billing for telehealth services and [ ] ways to safeguard Medicare from fraud, waste, and abuse related to telehealth.”[43] According to HHS OIG, at the outset of the COVID-19 pandemic, the use of telehealth—which preceded the pandemic—increased dramatically while, at the same time, the government temporarily paused program integrity efforts for Medicare, such as claims reviews. The study’s focus was on providers that billed for telehealth services and particularly those providers “whose billing for telehealth services poses a high risk to Medicare.”
The study established a number of criteria suggesting fraud, waste, or abuse, which HHS OIG used to determine providers that posed a high risk to the Medicare program and “warrant further scrutiny.” The seven criteria are: (1) “billing both a telehealth service and a facility fee for most visits”; (2) “billing telehealth services at the highest, most expensive level every time”; (3) “billing telehealth services for a high number of days in a year”; (4) “billing both Medicare fee-for-service and a Medicare Advantage plan for the same service for a high proportion of services”; (5) “billing a high average number of hours of telehealth services per visit”; (6) “billing telehealth services for a high number of beneficiaries”; and (7) “billing for a telehealth service and ordering medical equipment for a high proportion of beneficiaries.” According to the report, the Centers for Medicare and Medicaid Services (CMS) will “follow up on the providers identified in [the] report.”
Similarly, a December 2022 report by HHS OIG focused on laboratory testing for “add-on tests” in conjunction with COVID‑19 tests, and “found that 378 labs billed Medicare Part B for add‑on tests at questionably high levels . . . compared to the 19,199 other labs [studied].”[44] The report details specific types of “add-on” tests and dollar figures associated with Medicare payments for them.[45]
Studies such as these serve several functions. On one level, they signal to the public that the government is serious about fraud, waste, and abuse enforcement in industries affected by the pandemic, and they leverage partner agency investigative and analytical resources to provide DOJ (and the private relator’s bar) with insights for aligning enforcement efforts with agency programmatic priorities. They also demonstrate that the development of data-driven enforcement actions requires significant commitments of time and resources at the client agency level—a reality that helps explain why civil enforcement has publicly seemed slower compared to criminal prosecutions. And they serve as a reminder that DOJ does not view pandemic-related stimulus programs as the limit of its enforcement efforts; rather, we can expect DOJ to wield the FCA in response to industry developments prompted by the pandemic, beyond simply using the statute to recover fraudulently obtained stimulus funds.
Meanwhile, public signs of DOJ’s FCA enforcement efforts related to pandemic relief have continued to appear. In September, as discussed above, DOJ announced a settlement with a bank for allegedly processing a Paycheck Protection Program (PPP) loan on behalf of an ineligible borrower.[46] The announcement is significant because PPP FCA cases have typically been brought against borrowers who submitted false information. This is the first public settlement with a PPP lender, signaling that DOJ’s investigations have not been limited to borrowers (and that this case may not be the last one against a lender).
3. FCA Amendments Act of 2021 Still Pending Floor Vote
The FCA Amendments Act of 2021 (S. 2428) reached the end of the legislative session without a vote, having stalled continuously since it was reported out of the Senate Judiciary Committee in November 2021. The bill, introduced in July 2021 by Senator Chuck Grassley (R-IA) and a bipartisan group of co-sponsors, proposed two significant changes to the FCA.[47] First, it would amend the materiality requirement by providing that the government’s continued payment of funds to a defendant after discovery of fraud is not determinative of a lack of materiality “if other reasons exist for the decision of the government with respect to such refund or repayment.” Second, the bill would change the standard of review for evaluating a relator’s objection to the government’s decision to dismiss an FCA action.
In July 2022, the Congressional Budget Office (CBO) issued a lukewarm score on the proposed amendments.[48] With respect to the materiality amendment, the CBO estimated that DOJ would “succeed in about three FCA cases each year that would not otherwise have been won,” which would result in increasing collections by about $145 million over the decade of 2022-2032. However, the CBO did not indicate whether it factored in the potential for prolonged litigation and discovery costs arising from the need for the government to prove other reasons for having continued payment of claims despite knowledge of fraudulent activity. The predicted increase in collections must also be viewed in light of the CBO’s estimates regarding the increased costs likely to result from the bill’s imposition of a heightened burden on the government when it decides to dismiss an FCA action over a relator’s objection. The CBO estimated costs of $15 million to implement the amended dismissal requirements over the next five years, assuming an “additional month of work” for each case. While the CBO stated that its conclusions were “subject to considerable uncertainty,” the report is far from a clear endorsement of the proposed legislation, and may help explain its failure to progress in the Senate.
It is possible that the Senate also is waiting to see how the Supreme Court will rule in United States ex rel. Polansky v. Executive Health Resources, Inc. (discussed in our Case Law Developments update below). Polansky presents a challenge to the government’s right to seek dismissal, over a relator’s objection, of an FCA action in which the government has declined to intervene. During oral argument in December, the Justices appeared supportive of the government’s dismissal authority and seemed likely to set a low threshold for dismissal. The Senate also may also now be looking beyond Polansky to the Supreme Court’s grant of certiorari in United States ex rel. Schutte v. SuperValu Inc. et al. As discussed below, that case challenges the relevance of a defendant’s subjective beliefs to the FCA’s scienter requirement. Although Schutte does not directly involve the FCA provisions at issue in the Grassley amendments, the interrelated nature of the statute’s materiality and scienter requirements means that the decision in Schutte still could affect the trajectory of the Grassley amendments and the extent to which the materiality related amendment in particular is viewed as a necessity.
4. Congress Extends Limitations Period on CARES Act Fraud Prosecutions to 10 Years
On August 5, 2022, President Biden signed into law two bills extending the statute of limitations for CARES Act anti-fraud actions.[49] The laws establish a 10-year statute of limitations period for “any criminal charge or civil enforcement action” alleging fraud related to the Economic Injury Disaster Loan (EIDL) program or the Paycheck Protection Program (PPP). The EIDL and PPP programs both sprung from the Coronavirus Air, Relief, and Economic Security Act (CARES Act) and provided loans and emergency grants during the pandemic.[50] With respect to PPP loans, the legislation appears aimed at financial technology firms and their lenders, which the House Committee on Small Business calculated account for up to 75% of loans connected to fraud.[51] Unlike bank-related fraud, which carries a 10-year statute of limitations, see 18 U.S.C. § 3282, loan fraud connected to financial technology carries the 5-year limitations period for wire fraud, see 18 U.S.C. § 3293. The new laws aim to reconcile that discrepancy.
While the amendments are styled as changes to the Small Business Act in particular, they could have an effect on uses of the FCA to combat COVID relief fraud—if, for example, DOJ succeeds in arguing that the amendments actually do operate to extend the FCA’s statute of limitations, or if in practical terms the amendments make it easier for DOJ to rely on SBA-led enforcement actions rather than use the FCA itself. While the FCA also permits actions up to 10 years after the date of the violation, that outer limit only applies where the government or a relator utilizes the provision that tolls the default 6-year statute of limitations for 3 years from the date on which the government learns of the alleged violation.
B. STATE LEGISLATIVE DEVELOPMENTS
There were no major developments with respect to state FCA legislation in the second half of 2022. HHS OIG provides incentives for states to enact false claims statutes in keeping with the federal FCA. HHS OIG approval for a state’s FCA confers an increase of 10 percentage points in that state’s share of any recoveries in cases involving Medicaid.[52] Such approval requires, among other things, that the state FCA in question “contain provisions that are at least as effective in rewarding and facilitating qui tam actions for false or fraudulent claims” as are the federal FCA’s provisions.[53] Approval also requires a 60-day sealing provision and civil penalties that match those available under the federal FCA.[54] Consistent with our reporting in prior alerts, the lists of “approved” and “not approved” state false claims statutes remain at 22 and 7, respectively.[55]
IV. CASE LAW DEVELOPMENTS
A. SUPREME COURT WEIGHS GOVERNMENT’S AUTHORITY TO DISMISS QUI TAM LAWSUITS AND AGREES TO HEAR CRITICAL SCIENTER ISSUE
The Supreme Court granted certiorari this month on a petition regarding the question of whether a defendant who adopts an objectively reasonable interpretation of a legal obligation runs afoul of the FCA’s requirement that the defendant act “knowingly.” United States ex rel. Schutte v. SuperValu Inc., 9 F.4th 455 (7th Cir. 2021), cert. granted, 2023 WL 178398 (U.S. Jan. 13, 2023); United States ex rel. Proctor v. Safeway, Inc., 30 F.4th 649 (7th Cir. 2022), cert. granted, 2023 WL 178393 (U.S. Jan. 13, 2023). As noted above, the FCA defines “knowingly” to mean that a person “(i) has actual knowledge of the information; (ii) acts in deliberate ignorance of the truth or falsity of the information; or (iii) acts in reckless disregard of the truth or falsity of the information.” 31 U.S.C. § 3729(b)(1)(A). In Safeco Insurance Co. of America v. Burr, 551 U.S. 47 (2007), which addressed the Fair Credit Reporting Act’s nearly identical scienter requirement, the Supreme Court determined that a person who acts under an incorrect interpretation of a relevant statute or regulation does not act with “reckless disregard” if the interpretation is objectively reasonable and no authoritative guidance cautioned the person against it. Safeco, 551 U.S. at 70.
The relators in SuperValu alleged that when defendant SuperValu sought Medicare and Medicaid reimbursements, it misrepresented its “usual and customary” drug prices to government health programs that use that information to set reimbursement rates. See 9 F.4th at 459. After interpreting the relevant regulations, SuperValu reported its retail cash prices as its usual and customary drug prices rather than the lower, price-matched amounts that it charged customers under its price-match discount drug program, through which SuperValu would match discounted prices of local competitors upon request from anyone purchasing. Id. While the court agreed with the relator that SuperValu should have reported its discounted prices, the court applied the Safeco approach and determined that SuperValu’s interpretation of the regulations was objectively reasonable and that there was no authoritative guidance that warned SuperValu away from its interpretation. Id. at 472. According to the Seventh Circuit, whether SuperValu believed that its interpretation of “usual and customary” drug prices was the correct interpretation of the regulation did not bear on the objectively reasonable analysis. Instead, the court explained that “[a] defendant might suspect, believe, or intend to file a false claim, but it cannot know that its claim is false if the requirements for that claim are unknown.” Id. at 468. In other words, the focus should be on whether the interpretation was objectively reasonable, not on the defendant’s subjective intent. See id. at 466. The court therefore found that SuperValu faced no liability under the FCA. Id. at 472. This decision aligns with every other circuit that has considered Safeco’s application to the FCA (i.e., the Third, Eighth, Ninth, and D.C. Circuits).
The Supreme Court also granted a petition for certiorari in Safeway, which the Court then consolidated with SuperValu. Safeway dealt with substantially the same issue and outcome: applying the Safeco approach to Safeway’s interpretation of “usual and customary” drug prices to determine whether Safeway had violated the FCA. 30 4th at 658–59. Applying its decision from SuperValu, the Seventh Circuit in Safeway also found no liability for Safeway under the FCA because it had adopted an objectively reasonable interpretation of the relevant regulations and there was no authoritative guidance. Id. at 660, 663.
After the relators petitioned for a writ of certiorari, the Supreme Court asked the federal government to weigh in. The Solicitor General’s office disagreed with the position adopted by the Seventh Circuit, insisting that it opens up the possibility that defendants may be aware that their interpretation of a regulatory provision is wrong, but still proceed with the noncompliant action as long as they can later assert a reasonable justification for their preferred interpretation of the regulation after the fact. See United States ex rel. Schutte v. Supervalu Inc., No. 21-1326, Brief for the United States as Amicus Curiae, at 11–12 (Dec. 6, 2022). Senator Grassley, one of the chief proponents of the FCA in Congress, had filed an amicus brief as well, claiming that the Seventh Circuit opens a “gaping hole” in the FCA and urging the Supreme Court to grant certiorari and overturn the decision. See United States ex rel. Schutte v. Supervalu Inc., No. 21-1326, Brief for Senator Charles E. Grassley as Amicus Curiae, at 23 (May 19, 2022).
The Supreme Court’s resolution of this issue in SuperValu will have significant consequences going forward for FCA defendants, like SuperValu, who often are accused of certifying compliance with complex regulatory schemes. Defendants frequently argue the so-called Safeco defense, and the Supreme Court’s treatment of that issue could clarify the strength and scope of that defense. The Court’s decision will provide necessary guidance on whether a defendant can be “reckless” toward a statute or regulation that is amenable to multiple interpretations, even if the defendant allegedly doesn’t subjectively believe that interpretation to be correct. Coming on the heels of the Supreme Court’s seminal 2016 decision in Universal Health Services v. United States ex rel. Escobar, 579 U.S. 176 (2016), which clarified and strengthened the FCA’s materiality standard, this will be an opportunity for the Court to round out its jurisprudence on key elements of the FCA by addressing the statute’s scienter standard. We will be watching closely as this critical case gets its day at the Supreme Court.
In December, the Supreme Court heard oral argument in United States ex rel. Polansky v. Executive Health Resources, Inc., 17 F.4th 376 (3d Cir. 2021), cert. granted, 142 S. Ct. 2834 (2022). As we noted in the 2022 Mid-Year Update, the Supreme Court’s rather unexpected decision to grant certiorari in this case should at least result in a clarified standard for district courts to apply to government requests to dismiss qui tam complaints.
Until the Court issues its ruling on Polansky, the circuits remain split as to the standard under which a district court may evaluate the government’s decision to dismiss relators’ cases over their objection. Some courts have concluded that the government may dismiss virtually any action brought on behalf of the government, with very little scrutiny. Polansky, 17 F.4th at 384–88. Other courts have determined that if the government does not intervene in a relator’s case, the government must first intervene in the lawsuit before seeking to dismiss it under Federal Rule of Civil Procedure 41(a)’s standard. Id. Yet another subset of courts have indicated that the government must have some reasonable basis for the decision to dismiss, and ostensibly apply a degree of scrutiny to dismissal decisions. Id.
At oral argument, the Justices seemed inclined to grant DOJ broad discretion to dismiss cases, which is both a necessary check on runaway whistleblower litigation brought in the government’s name and a constitutional prerequisite to ensure the qui tam provision does not run afoul of constitutional limits on the executive branch’s ability to delegate its authority.
Regardless of how the Court resolves Polansky, however, the outcome is unlikely to have any immediate or substantial impact on the routine course of qui tam actions. In practice, district courts almost always agree to dismiss cases when DOJ seeks dismissal, regardless of what jurisdiction they are in and what standard they apply. In any event, we will be watching carefully to see whether the Supreme Court strengthens—or weakens—DOJ’s ability to reign-in qui tam lawsuits.
B. SUPREME COURT LEAVES IT TO CIRCUIT COURTS TO DEVELOP PLEADING STANDARDS
1. Supreme Court Denial of Cert Regarding Circuit Split on Rule 9(b)
Another important Supreme Court decision regarding the FCA in the past six months was a decision not to act, as it denied petitions for certiorari in three cases addressing similar questions that the petitioner claimed would have provided clarity on the appropriate pleading standard under Rule 9(b) of the Federal Rules of Civil Procedure for claims brought under the FCA. See Johnson v. Bethany Hospice and Palliative Care LLC, 143 S. Ct. 351 (2022); Molina Healthcare of Illinois, Inc. v. Prose, 143 S. Ct. 352 (2022); United States ex rel. Owsley v. Fazzi Assocs., Inc., 143 S. Ct. 362 (2022).
Bethany Hospice, Molina, and Owsley all dealt with a similar issue: how much specificity must a plaintiff provide in a complaint in an FCA case to meet the standards for alleging fraud under Rule 9(b)? In Bethany Hospice, the Eleventh Circuit made clear that to satisfy Rule 9(b) “a complaint must allege actual submission of a false claim, and . . . it must do so with some indicia of reliability.” 853 F. App’x 496, 501 (11th Cir. 2021) (internal quotation marks omitted). The relators had alleged that the defendant—a company providing for-profit hospice care—ran an illegal referral scheme, paying remuneration to physicians who referred Medicare patients to the defendant’s facilities. Id. at 496. The Eleventh Circuit determined that the relators had not adequately alleged an FCA violation because they failed to allege any details about specific representative false claims. Id. at 501–03.
In Owsley, the Sixth Circuit likewise dismissed a complaint from a relator that the defendants had submitted false data to the government in relation to Medicare claims for home-healthcare because the relator had provided insufficient details to allow the defendants to discern which claims they submitted were allegedly false. 16 F.4th 192, 194 (6th Cir. 2021). In doing so, the Sixth Circuit articulated a similar standard as in Bethany Hospice, explaining that “under Rule 9(b), ‘[t]he identification of at least one false claim with specificity is an indispensable element of a complaint that alleges a False Claims Act violation.’” Id. at 196 (alteration in original) (citation omitted).
In Molina, the relator alleged that Molina—who contracted with Illinois’s state Medicaid program—submitted false claims to receive capitation payments from the state for skilled nursing facility services under an implied false certification theory. 17 F.4th 732, 736–39 (7th Cir. 2021). There, the Seventh Circuit set forth a different standard for satisfying Rule 9(b), allowing the relator to proceed past a motion to dismiss where the relator “provide[d] information that plausibly support[ed] the inference that” the defendant submitted a false claim, even without the details of a specific false claim. Id. at 741. Even without allegations about a specific false claim, the Seventh Circuit determined that the circumstantial evidence the relator alleged created an inference that the defendants had submitted false claims. Id.
The standards adopted by the various circuits under Rule 9(b) exist on a spectrum, ranging from the Eleventh Circuit and Sixth Circuit—which have held that details of a specific false claim are required (i.e., the who, what, when, where, and how of the alleged fraudulent submissions to the government)—to the Seventh Circuit (and others such as the Third, Fifth, Ninth, Tenth, and D.C. Circuits) which have held that Rule 9(b) may be satisfied if the relator makes specific factual allegations as to a scheme to defraud and facts constituting reliable indicia that false claims resulted from the scheme. In Bethany Hospice, Prose, and Owsley, the petitioners sought guidance from the Supreme Court on the proper standard courts should apply when evaluating FCA claims under Rule 9(b). By denying the petitions for writ of certiorari in Bethany Hospice, Prose, and Owsley, the Supreme Court has effectively declined to resolve this circuit split at the present juncture and as a result has left open the possibility that plaintiffs will forum‑shop for the most favorable pleading standard when pursuing FCA cases.
2. Circuit Courts Continue to Craft Pleading Standards Under Rule 9(b)
Absent guidance from the Supreme Court, circuit courts continue to craft their own standard under Rule 9(b) in FCA cases. Three recent examples are illustrative.
In Lanahan v. County of Cook, 41 F.4th 854 (7th Cir. 2022), the Seventh Circuit was tasked with applying Rule 9(b) to various allegations by a former employee of the Cook County Department of Public Health (CCDPH). Id. at 858. After the government declined to intervene, the relator alleged in a complaint that the CCDPH had received federal grants to implement various federal initiatives in Cook County. See id. at 858–60. The relator further alleged that in distributing and accounting for the funds, the CCDPH had failed to follow federal guidelines and regulations. Id. The district court dismissed a first amended complaint and second amended complaint from the relator, determining that the relator had failed to adequately allege that the CCDPH had made any false claims to the federal government and failed to adequately connect any allegedly false statements to government payments. Id. at 860–61.
The Seventh Circuit affirmed the district court’s dismissal with prejudice, explaining that the relator had provided no more than conclusory assertions that the CCDPH submitted false claims to the government. Id. at 862–64. According to the Seventh Circuit, a major flaw in the relator’s claims was that for each of the payments she alleges violated the FCA, she “object[ed] only to Cook County’s treatment of the funds after they were disbursed. The Second Amended Complaint is utterly silent as to the events leading up to Cook County’s receipt of these funds.” Id. at 862. And while the relator had provided slightly more detail as to CCDPH’s alleged misuse of one category of funds set to be used to support providing H1N1 vaccinations—by specifically alleging that CCDPH submitted falsified expense reports—these additional details were still not enough to satisfy Rule 9(b) because the relator did “not support this claim with particularized information about how . . . the expense reports [were] prepared.” Id. at 863. The Seventh Circuit further determined that the Relator had failed to allege adequate facts under Rule 9(b) to connect the allegedly false statements to the government payments. Id. at 864.
The Seventh Circuit had a further opportunity to provide guidance on the application of Rule 9(b) in United States ex rel. Sibley v. University of Chicago Medical Center, 44 F.4th 646 (7th Cir. 2022). While the federal and state governments elected not to intervene, the relators—former employees of jointly owned companies that deliver medical billing and debt collection services to healthcare providers—claimed their former employers (debt collection companies) and the healthcare provider those debt collection companies serviced had failed to follow federal regulations governing “bad debt.” Id. at 651. “Bad debts” are incurred when a Medicare patient fails to make required deductible or coinsurance payments and the provider makes sufficient efforts to collect the debt. Id. at 652 (citing 42 C.F.R. § 413.89). The provider may seek reimbursement from the Center for Medicare and Medicaid Services (CMS) for those bad debts. 44 F.4th at 652. The relators alleged that the debt collection agencies and the healthcare provider had failed to follow the federal regulations for what constitutes bad debt in seeking reimbursements from CMS, and thus violated the FCA by failing to repay the government excess reimbursements received for bad debt. See id. at 652–655 The relators also alleged they had been retaliated against by the companies for reporting the alleged FCA violations. Id.
The district court dismissed all of the relators’ claims against the former employers and the healthcare provider for failure to adequately state a claim under Rule 9(b). Id. at 655. The Seventh Circuit affirmed the dismissal of most of the claims. The Seventh Circuit first explained that the complaint failed to allege that the healthcare provider had direct knowledge of the alleged excessive reimbursements—mere inferences and assumptions did not suffice to show knowledge. Id. at 657–58. Next, the court explained that for claims premised on the failure to repay the government for excessive bad debt reimbursements, the relators must provide “specific representative examples” of false claims. Id. at 659. According to the Sibley court, the relator failed to provide such representative examples for one of the debt collection companies and thus dismissal as to that company was appropriate. Id. In reaching this conclusion, the Sibley court appears to be at odds with the Seventh Circuit’s decision in Molina, in which the court noted that the plaintiff “provide[d] information that plausibly supports the inference that [the defendant] included false information” in submissions to the government, even without specific details of the submissions. 17 F.4th at 741. In Sibley, the Seventh Circuit went on to reverse the district court’s decision to dismiss the complaint against the other debt collection company because the relators had provided three specific examples of bad debt that was allegedly improperly reimbursed. 44 F.4th at 660. Finally, the Seventh Circuit also allowed the relators’ retaliation claims to proceed, making clear that those claims were governed by Rule 12(b)(6)’s pleading standard rather than the more stringent Rule 9(b) standard. Id. at 661–62.
The Fourth Circuit also addressed Rule 9(b)’s pleading standard in United States ex rel. Nicholson v. MedCom Carolinas, Inc., 42 F.4th 185 (4th Cir. 2022). In Nicholson, the federal government declined to intervene in a case involving allegations that the defendant—who contracted with the manufacturer of skin grafts to sell them to hospitals—paid its salespeople a commission for the skin grafts sold to federal healthcare providers, including Veterans Administration (VA) hospitals. Id. at 189. The relator then prosecuted the case. Id. According to the relator, selling the skin grafts to VA hospitals on commission resulted in a violation of the Anti-Kickback Scheme (AKS), which in turn led to a violation of the FCA. Id. The district court dismissed the relator’s complaint under Rule 9(b), determining it was almost entirely conclusory and provided no meaningful details to support the claims. Id.
The Fourth Circuit affirmed the district court’s dismissal. Id. at 200. The court explained that under Rule 9(b), the relator either had to provide a representative example of an alleged false claim (including the “time, place, and contents of the misrepresentation”) or make allegations sufficient to show that the defendant was engaged in “a pattern of conduct that would necessarily have led to the submission of false claims.” Id. at 194 (citations and internal quotation marks omitted). The Fourth Circuit concluded that the relator had not alleged either. Id. at 196. Specifically, while the relator had pled a specific false claim, the complaint lacked any details: “The patient is unknown . . ., who submitted the claim is unknown . . ., what VA hospital in what state is unknown . . . . The unknowns swamp the knowns.” Id. Given the bare‑bones details included in the complaint, the Court concluded it lacked the particularity required under Rule 9(b).
C. THE D.C. CIRCUIT APPLIES PRO TANTO APPROACH TO MULTI-DEFENDANT FCA CASE
In United States v. Honeywell International Inc., 47 F.4th 805 (D.C. Cir. 2022), the D.C. Circuit was faced with an unresolved damages-related question under the FCA, namely whether the statute provides, in multi-defendant cases, for an offset of previous settlement recoveries against a non-settling joint tortfeasor’s liability. Many FCA cases involve multiple defendants, in which those defendants are subject to joint and several liability. Honeywell clarified that in such cases, the pro tanto rule applies: proceeds from settlements with joint tortfeasor defendants should reduce the amounts owed by other, non-settling defendants—at least in regard to compensatory damages. However, the case left open whether civil penalties could qualify for such an off-set between joint tortfeasors in FCA cases.
The Honeywell appeal stemmed from a suit brought by the federal government against Honeywell, based on alleged FCA violations. According to the government, Honeywell had misrepresented the quality of a material it manufactured and provided to bulletproof vest manufacturers who eventually sold them to the government. According to the government, Honeywell had improperly represented that the material it sold to the manufacturers was the “best ballistic product in the market for ballistic resistance,” despite the fact that the materials degraded at high temperatures. Id. at 810. While the government’s suit against Honeywell was ongoing, the government settled with several other parties involved in manufacturing and supplying the vests to the federal government. Honeywell moved for summary judgment on the issue of damages, claiming that any damages assessed against it should be offset based on the settlements with the other parties. Honeywell “maintained the court should apply a pro tanto approach, reducing any common damages Honeywell owed by the amount of the settlements.” Id. at 811. The district court, however, adopted the proportionate share method for calculating damages advocated for by the government, which meant that “Honeywell would still be responsible for its proportionate share of the $35 million” in claimed damages. Id.
On appeal, the D.C. Circuit acknowledged that “[t]he FCA says nothing at all about how to address indivisible harms or whether joint and several liability is appropriate.” Id. at 813. Faced with crafting a common law rule, the D.C. Circuit determined that the “pro tanto rule . . . is not just compatible with the FCA; it is a better fit with the statute and the liability rules that have been partnered with it.” Id. at 817. The D.C. Circuit recognized that allowing for the pro tanto rule to be applied in FCA cases would mean that if settlements exceeded the damages claimed by the government, a defendant—like Honeywell—would potentially face no damages. But the D.C. Circuit still believed the pro tanto rule was “consistent with the FCA” because it left the “government in the driver’s seat to pursue and punish false claims according to its priorities.” Id. at 818. The D.C. Circuit’s decision represents the first circuit court decision on this issue and provides important reasoning for later courts that may be faced with determining how much settlements with third parties may offset damages against defendants in FCA cases. The case also will affect how defendants and the government answer the difficult question of how an individual defendant should value a potential settlement in a multi-defendant case. Defendants will have to decide whether to wait out resolutions with others that could reduce their own exposure, while the government will need to decide whether to reduce its settlement demands of earlier-settling parties in order to leave some amount of damages on the table to incentive later-in-time defendants to settle.
D. THE EIGHTH CIRCUIT CREATES A CIRCUIT SPLIT ON CAUSATION FOR AKS-PREDICATED FCA CLAIMS
The AKS imposes criminal liability on a person who knowingly and willfully pays, offers, solicits, or receives remuneration in return for referrals or orders of items or services reimbursed by federal health programs.[56] In 2010, Congress amended the AKS to provide that “a claim that includes items or services resulting from a violation of [the AKS] constitutes a false or fraudulent claim for purposes of [the FCA].” DOJ has long asserted the position that the term “resulting from” does not require a showing of causation; instead, DOJ asserts in AKS-based FCA cases that every claim that came later in time than the receipt of a kickback is “tainted” by the kickback and therefore is a false claim. In United States ex rel. Cairns v. D.S. Medical LLC, 2022 WL 2930946 (8th Cir. July 26, 2022), however, the Eighth Circuit held that the appropriate causation standard for AKS‑based FCA liability was “but for” causation. Cairns created a growing circuit split on the question of which claims “result from” an AKS violation. For example, in United States ex rel. Greenfield v. Medco Health Solutions, Inc., 880 F.3d 89, 98 (3d Cir. 2018), the Third Circuit, rejected both the “but for” causation standard and DOJ’s preferred “taint” theory, instead findings that the FCA and AKS “require[] the much looser standard of showing a link in the causal chain.” In Cairns, relators brought a qui tam action against a neurosurgeon, his practice, his fiancée, and the spinal implant company his fiancée owned, alleging a kickback scheme between the couple resulting in FCA violations. The relator alleged that a physician ordered spinal implants from his fiancée’s company—which allegedly received large commissions from the implant manufacturers—in exchange for an offer to purchase that company’s stock. The United States filed its own complaint as an intervenor. Cairns, 42 F.4th at 831–33. In a jury trial before the district court, the government argued that the 2010 amendment to the AKS created a loose FCA causation standard such that the alleged kickbacks would “taint” the claims and cause an FCA violation. See id. at 833–35. The district court issued jury instructions that the government could establish falsity if it showed “that the claim failed to disclose the Anti-Kickback Statute violation.” Id. at 834 (modifications omitted). The jury found for the government and the district court awarded damages and penalties in excess of five million dollars. Id. at 832. Defendants appealed, and the Eighth Circuit remanded. Id. On remand, the district court granted the government’s motion to dismiss its remaining claims without prejudice. Id. Defendants appealed again, arguing the lower court’s jury instructions were defective in failing to instruct the jury on but for causation. Id.
The Eighth Circuit agreed. In a noteworthy rejection of the government’s position, the Eighth Circuit held the plain meaning of the AKS required a showing of but for AKS-to-FCA causation— essentially, a showing that but for the alleged kickback, the FCA claim at issue would not have included the alleged kickback’s “items or services.” Id. at 836. The Eighth Circuit chiefly based its reasoning on the Supreme Court’s analysis of similar “results from” statutory language in the Controlled Substances Act in Burrage v. United States, 571 U.S. 204, 210–11 (2014). In Burrage, the Court reasoned that “results from” in the phrase “death or serious bodily injury results from the use of [the] substance” requires a showing of actual causality, or but for causation. Id. at 209 (citing 21 U.S.C. § 841(b)(1)(A)-(C)). Likewise, the Eighth Circuit reasoned in Cairns that the plain meaning of “resulting from” was “unambiguously causal,” rejecting the government’s arguments that causation could be shown where the kickback “tainted” the claim or was a “contributing factor.” Cairns, 42 F.4th at 835–36. The alternative standards were “hardly causal at all” – a “‘taint’ could occur without the illegal kickbacks motivating the inclusion of any of the ‘items or services’” and “asking the jury if a violation ‘may have been a contributing factor’ does not establish anything more than a mere possibility.” Id. at 835. Worst of all, the Eighth Circuit added, was the district court’s instruction that “may have been the least causal of all: just because a claim fails to disclose an anti-kickback violation does not mean that there is a connection between the violation and the included ‘items or services.’” Id. The Eighth Circuit explained that, “[w]here there is no textual or contextual indication to the contrary, courts regularly read phrases like ‘results from’ to require but-for causality.” Id. (citation omitted).
E. THE FOURTH AND NINTH CIRCUITS ISSUE DECISIONS ON THE SCIENTER REQUIRED UNDER THE FCA
In United States ex rel. Hartpence v. Kinetic Concepts, Inc., 44 F. 4th 838 (9th Cir. 2022), the Ninth Circuit confronted the FCA’s scienter requirement. A relator alleged that a wound care medical device manufacturer and its subsidiary falsely certified compliance with Medicare payment rules about the use of the medical devices. Id. at 841, 844–45. The United States declined to intervene. Id. at 844–45. The relator claimed that the defendants fraudulently certified compliance with Medicare reimbursement criteria that required that the medical records of patients who used the devices reflect “progressive wound healing” for each month for which claims were submitted. Id. at 841. The relator alleged that the defendants manipulated their billing codes to falsely certify compliance during “stalled cycles” of months without healing, where healing resumed the next month. Id. at 844–45.
The district court granted summary judgment in favor of the defendants, holding that relator had not brought sufficient evidence that the defendants’ false certifications were material to the Medicare reimbursements or that the defendants had knowingly used the billing codes as alleged. Id. at 845. The relator appealed. Id.
The Ninth Circuit reversed, holding that the district court erred in so ruling because the relator produced enough evidence to raise a triable issue of fact regarding the requisite scienter. Id. at 850–53. The court explained that based on the relator’s evidence, a jury could find that the defendants deliberately miscoded claims to conceal them and knew those coded certifications were false for two reasons. Id. at 851. First, the relator put forth evidence, in the form of the defendants’ internal communications, that suggested the defendants deliberately used the codes fraudulently to skirt claim appeals and denials. Second, the relator brought evidence both of the defendants’ employees raising concerns internally about the billing and of Medicare contractors correcting defendants’ application of the billing codes. Id. Although the defendants did not automatically accept coded claims as true, there was email communication evidence in the record that they were “plainly aware that using the [] modifier avoided a costly review and appeals process that it would sometimes win and sometimes lose.” Id. That evidence – which the court suggested showed the defendants deliberately avoided digging into the validity of claim modifiers – provided “ample evidence to permit a rational trier of fact to conclude that [the defendants] knew that it was a false statement . . . and that [the defendants] did so knowing that it might thereby escape case-specific scrutiny.” Id. at 851–52.
An en banc Fourth Circuit examined the FCA’s scienter element in United States ex rel. Sheldon v. Allergan Sales, LLC, 24 F.4th 340 (4th Cir. 2022), vacated en banc, 49 F.4th 873 (4th Cir. 2022). The relator alleged that the drug manufacturer falsely represented its drugs’ “best price” under the Medicaid drug rebate program by purportedly failing to aggregate discounts given to separate customers. Id. at 346. The government declined to intervene. Id. The district court granted the manufacturer’s motion to dismiss, holding that the relator had “failed to plead both that the claims at issue were false and that [the manufacturer] had made them knowingly.” Id. at 346–47.
In Sheldon, the divided Fourth Circuit panel joined the growing number of circuits to address the Supreme Court’s Safeco scienter standard in FCA cases. Id. at 347. The court noted the difficulty of applying the vague “knowledge” standard set forth in the FCA. The court held that “a defendant cannot act ‘knowingly’ if it bases its actions on an objectively reasonable interpretation of the relevant statute when it has not been warned away from that interpretation by authoritative guidance” – an “objective standard” that precludes inquiry into a defendant’s subjective intent. Id. at 348. However, shortly after oral argument and in a per curiam order on rehearing en banc, the full Fourth Circuit vacated the panel opinion and affirmed the district court. United States ex rel. Sheldon v. Allergan Sales, LLC, 49 F.4th 873, 874 (4th Cir. 2022).
F. THE NINTH AND FOURTH CIRCUITS ADDRESS THE BOUNDS OF THE PUBLIC DISCLOSURE BAR
In United States ex rel. Silbersher v. Allergan, Inc. et al., 46 F.4th 991 (9th Cir. 2022), the Ninth Circuit considered the FCA’s “public disclosure bar,” which directs a district court to dismiss an action under the FCA when “substantially the same allegations or transactions” have previously been publicly disclosed, unless the relator is an “original source of the information.” 31 U.S.C. § 3730(e)(4)(A). In a qui tam action, a patent attorney alleged that the defendant drug companies had improperly obtained patents to protect two of their drugs from generic competition. Id. at 993. The government declined to intervene. Id. The district court denied the defendants’ motion to dismiss, and Defendants appealed.
On appeal, the Ninth Circuit—interpreting the revised 2010 public disclosure bar for the first time—reversed and remanded, holding that the district court erred in concluding that the relator’s case did not trigger the FCA’s public disclosure bar. The Ninth Circuit re-emphasized that its public disclosure bar test has three elements: “‘(1) the disclosure at issue occurred through one of the channels specified in the statute; (2) the disclosure was “public”; and (3) the relator’s action’ is substantially the same as the allegation or transaction publicly disclosed.” Id. at 996 (citation omitted). The court explained that only the first element, what constitutes a channel for disclosure under the FCA, was at issue in the case. Id. The court agreed with the defendants that the relator’s claims were barred because the underlying information came from a public patent prosecution, which is an “other Federal . . . hearing.” Id. at 999 (internal quotation marks omitted). Because the district court had not decided whether the relator was an “original source” such that he fell under that exception to the public disclosure bar, the Ninth Circuit remanded the case to the district court. Id. at 1000 (internal quotation marks omitted).
The Second Circuit came to a similar conclusion in United States ex rel. CKD Project, LLC v. Fresenius Medical Care Holdings, Inc., 2022 WL 17818587 (2d Cir. Dec. 20, 2022), finding the public disclosure rule barred a relator’s suit because the material information of the relator’s claim had been publicly disclosed in the defendant’s securities filings and because the relator did not fall into the original source exception. The relator alleged that the company performed unnecessary procedures on dialysis patients and fraudulently submitted claims to Medicare, Medicaid, and state health programs. Id. at *1–3. The United States declined to intervene. Id. at *1. The district court dismissed relator’s claims without leave to amend. Id. at *2.
The Second Circuit affirmed, explaining both that (1) the relator’s claims fell under the public disclosure bar because the “critical or material elements” of the transactions were already publicly disclosed and (2) the relator did not fall under the original source exception because it “d[id] not possess direct knowledge of the information on which the allegations are based” and was an “entity formed solely for this litigation.” Id. at *3. While the relator argued the defendant’s filings did not contain material information about the details of an acquisition and shell entity scheme alleged in the claim, the court explained that the additional details the relator highlighted were just that—details—and the material elements of the acquisitions were publicly disclosed. Id. at *4. The Second Circuit found that because the core elements of the claim were in Fresenius’ securities filings, they had been publicly disclosed. Id.
G. THE TENTH AND SECOND CIRCUITS APPLY THE APPROACH TO FCA MATERIALITY DESCRIBED BY THE SUPREME COURT IN ESCOBAR
In United States ex rel. Sorenson v. Wadsworth Brothers Construction Co., 48 F.4th 1146 (10th Cir. 2022), a relator alleged that Wadsworth Brothers Construction Company violated the FCA by falsely certifying its compliance with prevailing-wage requirements under the Davis-Bacon Act, 40 U.S.C. §§ 3141–38. The government declined to intervene and the district court dismissed Sorenson’s claims based on his failure to satisfy the “demanding materiality standard” established in Escobar, and the Tenth Circuit affirmed. Id. at 1155.
In 2012, the Salt Lake International Airport received a $9 million federal grant to make improvements. Id. at 1154. It solicited bids for construction of a deicing pad, and noted in its bid-solicitation documents that the winning contractor would be required to certify its compliance with the Davis-Bacon Act. Id. Wadsworth, as the lowest bidder, entered into a contract with the Airport and began construction in 2013. Id.
The relator worked on the project as a truck driver for Wadsworth for just over two months in 2014. He alleged that he was underpaid during that time as his pay “reflected substantial work on non-Davis-Bacon jobsites,” despite having worked “exclusively” on Wadsworth’s “federally funded” projects. Id. at 1154. According to the relator, Wadsworth “represented to the federal government on each of its invoices involving [the relator] that the wages Wadsworth paid [him] complied with the Davis-Bacon Act,” and did so “despite actually knowing it did not pay Sorenson in accord with applicable Davis-Bacon requirements,” thus causing the government to pay Wadsworth more than it was entitled to receive. Id. at 1155. The district court dismissed the claims on grounds that the only basis for a finding of materiality was “that certification of compliance with the Davis-Bacon Act is a prerequisite to the payment” of Wadsworth’s invoices, and as Escobar clarified, “‘minor or insubstantial’ noncompliance with statutory, regulatory, or contractual requirements” is “not enough, standing alone, to render a misrepresentation material,” whether or not the requirement is designated with the terminology of a condition of payment. Id. at 1152 (quoting Escobar, 579 U.S. at 194).
The Tenth Circuit affirmed what it considered to be a relatively ordinary decision after Escobar, noting that the “court need not make any grand pronouncements about the general materiality of Davis-Bacon violations to resolve Sorenson’s appeal.” Id. at 1156. Acknowledging that Davis-Bacon wages are determined by jobsite and task, rather than by project, the court found that the relator had failed to allege any of those context-specific factors that would allow for a finding of materiality. The complaint failed to identify the relevant Davis-Bacon jobsites, establish that relator worked at those jobsites, or even demonstrate that his work as a truck driver was covered under the Davis-Bacon Act. It therefore was “bereft of details from which any estimate of the quantum of alleged underpayments could be made, and thus there is no indication as to whether the amount involved is minor or significant.” Id. at 1157. The court further affirmed the district court’s grant of summary judgment on a retaliation claim brought by the relator. Id. at 1158.
In United States ex rel. Yu v. Grifols USA, LLC, 2022 WL 7785044 (2d Cir. Oct. 14, 2022), a relator alleged that pharmaceutical manufacturer Grifols USA, and associated entities Grifols Biologicals, Grifols, S.A., and Grifols Shared Services (collectively, “Grifols”) fraudulently obtained FDA approval of Gamunex, one of its products designed to treat various autoimmune disorders, and thus submitted false claims to the government for payment of various “Government Healthcare Programs” related to Gamunex. The government declined to intervene and the district court dismissed the action for failing to sufficiently plead materiality, and the Second Circuit affirmed. Id. at *2.
Before producing Gamunex, Grifols was required to obtain a Prior Approval Supplement from the FDA by having the FDA inspect its manufacturing facilities and equipment for compliance with current Good Manufacturing Practices (cGMPs) required by FDA regulations. Id. at *1. Grifols hired the relator to perform regular quality assurance inspections its Gamunex plant. The relator alleged that the plant was not operating in accordance with applicable cGMPs but that Grifols certified otherwise to the FDA, thus fraudulently obtaining approval by the FDA to manufacture and sell Gamunex. Id.
The Second Circuit affirmed the district court by summary opinion. The relator failed to establish any contractual provision expressly conditioning government payment on Grifols’ compliance with specific cGMPs or that the FDA violations he claimed to have witnessed “resulted in ‘significant financial cost to the government,’ or that the violations go to the ‘heart of the bargain.’” Id. at *5. Accordingly, the relator did “not plausibly allege that any misrepresentation by Grifols materially impacted the Government Healthcare Programs’ payment determination.” Id.
In Lee v. Northern Metropolitan Foundation for Healthcare, Inc., 2022 WL 17366627 (2d Cir. Dec. 2, 2022), relators alleged that defendants, state and federally funded operators of an adult day healthcare program, discriminated against its registrants on the basis of national origin and provided them with substandard care. The relators alleged that the defendants thus violated the FCA by impliedly certifying compliance federal and state anti-discrimination laws and medical-care standards when submitting claims to the government for reimbursement. Id. at 2. The state and federal governments declined to intervene and the case proceeded to trial. After a bench trial, the district court entered judgment against relators pursuant to FRCP 52(a) based on the relators’ failure to establish materiality. Id. at *3 n.2. The Second Circuit affirmed by summary order.
The relators argued on appeal that the evidence demonstrated materiality under the Escobar factors, and in the alternative, materiality was evident based on the “common-sense notion that violations of allegedly important statutes and regulations pertaining to discrimination and medical-model facilities would have affected a reasonable administrator’s decision to pay” defendants’ medical claims. Id. at *1-2.
The Second Circuit rejected both arguments. Applying Escobar, the court found that the relators “adduced no evidence that compliance with the anti-discrimination and medical-model statutes and regulations at issue” was a condition of payment, “no evidence concerning the government’s response” to defendants’ alleged noncompliance, and “little evidence from which one could conclude that the discrimination and medical-model infractions at Northern undermined ‘the essence of the bargain’” between defendants and the government. Id. at 2 (emphases in original) (quoting United States ex rel. Foreman v. AECOM, 19 F.4th 85, 116-17 (2d Cir. 2021)). The court also found the relators’ common-sense argument unpersuasive, explaining that while common sense “may have a role” in assessing materiality, this case did not provide such an opportunity, “as here, there is not a tight fit between the implicit misrepresentation and the service provided.” Id.
H. THE ELEVENTH AND THIRD CIRCUITS EVALUATE RETALIATION CLAIMS BROUGHT UNDER THE FCA
In Simon ex rel. Florida Rehabilitation Associates PLLC v. Healthsouth of Sarasota Ltd. Partnership, 2022 WL 3910607 (11th Cir. Aug. 31, 2022), a relator asserted a retaliation claim under the FCA alleging that she suffered various adverse employment actions and was ultimately constructively discharged after complaining to defendants about allegedly false medical diagnoses. After the relator filed a qui tam action in 2012 alleging various acts of fraud against the government, the United States intervened and reached a settlement with HealthSouth, at which point the relator, the government, and defendants stipulated to dismissal with prejudice of all FCA claims except for relator’s retaliation claim. Id. at *3-4. The relator proceeded to litigate her retaliation claim until the district court granted summary judgment to the defendants. Id. at *1. The Eleventh Circuit affirmed.
The relator, a physiatrist, operated an outpatient medical practice through Florida Rehabilitation Associates and worked as an attending physician at defendant HealthSouth Sarasota Hospital. Id. at *2. The relator claimed that defendants directed her and other physicians to diagnose patients with “disuse myopathy,” which she believed was a “fraudulent diagnosis” created by HealthSouth to inflate the number of patients it treated for certain severe conditions so that it could classify as an “inpatient rehabilitation facility” entitled to CMS funding. Id. at *2; see 42 C.F.R. § 412.29(b)(1)–(2). The relator alleged that she was threatened, demoted, and investigated after making numerous verbal complaints about these diagnoses. Id.
The Eleventh Circuit affirmed judgment against the relator and agreed with the district court that she had not engaged in a statutorily protected activity. Id. at *6. Citing to another recent case within its circuit, Hickman v. Spirit of Athens, Ala., Inc., 985 F.3d 1284, 1287 (11th Cir. 2021), the court “assumed without deciding” that the plaintiff in an FCA retaliation case “must at least show that she had an objectively reasonable belief that her employer violated the FCA to establish that she engaged in protected activity,” and determined that the relator here was unable to do so. Id. Although the evidence demonstrated “that [the relator] possessed a sincere, subjective belief that HealthSouth was committing fraud by using a fabricated disuse myopathy diagnosis,” the relator failed to meet the burden of showing that her belief was objectively reasonable. Id. The relator testified to her own belief of the illegitimacy of the diagnosis, but “she offered no evidence that she had an objectively reasonable belief that the doctors who diagnosed their patients with disuse myopathy did so purposefully and wrongly to fraudulently receive money from the government,” and thus she could not establish that she engaged in statutorily protected conduct. Id. at *7.
In United States ex rel. Ascolese v. Shoemaker Constr. Co., 55 F.4th 188 (3d Cir. 2022), a relator asserted an FCA retaliation claim arising out of conduct that took place during a federally funded construction project. The case presented the Third Circuit with its first opportunity to clarify the standard for retaliation under the FCA since 2009-2010 congressional amendments to whistleblower protections under the Act. After the government declined to intervene in the action, relator amended the complaint to remove those claims which applied only to the government, and the court dismissed the amended complaint without prejudice. Id. at 193. The relator moved for leave to file a second amended complaint and the district court denied the motion on grounds that amendment would be futile due to the relator’s failure to show that defendants had adequate notice of his FCA complaints. Id. The Third Circuit disagreed, vacating the judgment and remanding the action to the district court for further proceedings.
The United States Department of Housing and Urban Development granted $30 million to the Philadelphia Housing Authority (PHA) to construct public housing in North Philadelphia. Id. at 191-92. The PHA designated defendants Shoemaker Construction Company and Shoemaker Synterra JV as construction managers for the project, who then subcontracted defendant McDonough Bolyard Peck Inc. (MBP) to perform quality control services and ensure that the project complied with all applicable construction regulations. Id. The relator worked as a Quality Assurance/Quality Control Manager for the project and was responsible for reporting any “deficiencies” in the project, such as design plans, specifications and building codes. Id. According to the relator, the relator noted “dozens” of deficiencies in the project and conveyed those deficiencies to defendants, who took no further action in response to his complaints. Id. The relator “broke his chain of command” and took his complaints directly to the PHA, which the relator alleged caused defendants to take retaliatory measures.
In finding that the relator had sufficiently pled retaliation, the Third Circuit explained that “the amendments to the anti-retaliation provision reflect a congressional intent to expand protection to ‘efforts to stop violations before they happen or recur.’” Id. at 194-95 (emphasis in original) (quoting Singletary v. Howard Univ., 939 F.3d 287, 296 (D.C. Cir. 2019)). The “fact intensive” inquiry into whether the relator did “more than his job responsibilities” to trigger FCA protection should require the district court to “focus on whether [the relator] acted outside of his chain of command or his job duties.” Id. The Third Circuit reasoned that the relator had done so by outlining his “usual job responsibilities” and establishing the “contours of his chain of command” in order to adequately allege that his actions had gone beyond the scope of those responsibilities by reporting his concerns to the PHA. Id. at 196. Thus, the relator sufficiently pled that he engaged in protected conduct. Furthermore, the relator plausibly alleged that MBP was on notice of his protected conduct and retaliated against him as a result, as it “was aware that [the relator] made external reports to the PHA,” and that such conduct was “outside of his reporting chain of command.” Id.
V. CONCLUSION
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2023 False Claims Act Mid-Year Update, which we will publish in July 2023.
________________________
[1] See U.S. Dep’t of Justice, Fraud Statistics Overview (Feb. 7, 2023), https://www.justice.gov/opa/press-release/file/1567691/download [hereinafter DOJ FY 2021 Stats].
[2] Id.
[3] Id.
[4] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, False Claims Act Settlements and Judgments Exceed $2 Billion in Fiscal Year 2022 (Feb. 7, 2022), https://www.justice.gov/opa/pr/false-claims-act-settlements-and-judgments-exceed-2-billion-fiscal-year-2022 [hereinafter DOJ FY 2022 Recoveries Press Release].
[5] Id.
[6] See DOJ FY 2021 Stats.
[7] See DOJ FY 2022 Recoveries Press Release
[8] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Fifteen Texas Doctors Agree to Pay Over $2.8 Million to Settle Kickback Allegations (June 28, 2022), https://www.justice.gov/opa/pr/fifteen-texas-doctors-agree-pay-over-28-million-settle-kickback-allegations.
[9] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, MorseLife Nursing Home Health System Agrees to Pay $1.75 Million to Settle False Claims Act Allegations for Facilitating COVID-19 Vaccinations of Ineligible Donors and Prospective Donors (June 30, 2022), https://www.justice.gov/opa/pr/morselife-nursing-home-health-system-agrees-pay-175-million-settle-false-claims-act.
[10] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Department of Justice Settles Lawsuit Against Spine Device Distributor and its Owners Alleging Illegal Kickbacks to Physicians (July 1, 2022), https://www.justice.gov/opa/pr/department-justice-settles-lawsuit-against-spine-device-distributor-and-its-owners-alleging.
[11] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, West Virginia Hospital to Pay $1.5 Million to Settle Allegations Concerning Impermissible Financial Relationships with Referring Physicians (July 7, 2022), https://www.justice.gov/opa/pr/west-virginia-hospital-pay-15-million-settle-allegations-concerning-impermissible-financial.
[12] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Solera Specialty Pharmacy Agrees to Enter Deferred Prosecution Agreement; Company and CEO to Pay $1.31 Million for Submitting False Claims for Anti-Overdose Drug (July 13, 2022), https://www.justice.gov/opa/pr/solera-specialty-pharmacy-agrees-enter-deferred-prosecution-agreement-company-and-ceo-pay-131.
[13] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, BioReference Laboratories and Parent Company agree to Pay $9.85 Million to Resolve False Claims Act Allegations of Illegal Payments to Referring Physicians (July 14, 2022), https://www.justice.gov/opa/pr/bioreference-laboratories-and-parent-company-agree-pay-985-million-resolve-false-claims-act.
[14] See Press Release, U.S. Atty’s Office for the Dist. of MA, Inform Diagnostics Agrees to Pay $16 Million to Resolve False Claims Act Allegations of Medically Unnecessary Tests (July 20, 2022), https://www.justice.gov/usao-ma/pr/inform-diagnostics-agrees-pay-16-million-resolve-false-claims-act-allegations-medically.
[15] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Medical Device Manufacturer Biotronik Inc. Agrees to Pay $12.95 Million to Settle Allegations of Improper Payments to Physicians (July 22, 2022), https://www.justice.gov/opa/pr/medical-device-manufacturer-biotronik-inc-agrees-pay-1295-million-settle-allegations-improper.
[16] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Metric Lab Services, Metric Management Services LLC, Spectrum Diagnostic Labs LLC, and Owners Agree to Pay $5.7 Million to Settle Allegations of False Claims for Unnecessary Genetic Testing (July 22, 2022), https://www.justice.gov/opa/pr/metric-lab-services-metric-management-services-llc-spectrum-diagnostic-labs-llc-and-owners.
[17] See Press Release, U.S. Atty’s Office for Central Dist. of CA, Metric Lab Services, Venture County’s Organized Health System and 3 Medical Providers Agree to Pay $70.7 Million to Settle False Claims Act Allegations (Aug. 18, 2022), https://www.justice.gov/usao-cdca/pr/ventura-county-s-organized-health-system-and-3-medical-providers-agree-pay-707-million#:~:text=August%2018%2C%202022-,Ventura%20County’s%20Organized%20Health%20System%20and%203%20Medical%20Providers%20Agree,Settle%20False%20Claims%20Act%20Allegations.
[18] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Essilor Agrees to Pay $16.4 Million to Resolve Alleged False Claims Act Liability for Paying Kickbacks (Aug. 23, 2022), https://www.justice.gov/opa/pr/essilor-agrees-pay-164-million-resolve-alleged-false-claims-act-liability-paying-kickbacks.
[19] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Philips Subsidiary to Pay Over $24 Million for Alleged False Claims Caused by Respironics for Respiratory-Related Medical Equipment (Sept. 1, 2022), https://www.justice.gov/opa/pr/philips-subsidiary-pay-over-24-million-alleged-false-claims-caused-respironics-respiratory.
[20] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Bayer to Pay $40 Million to Resolve the Alleged Use of Kickbacks and False Statements Relating to Three Drugs (Sept. 15, 2022), https://www.justice.gov/opa/pr/bayer-pay-40-million-resolve-alleged-use-kickbacks-and-false-statements-relating-three-drugs.
[21] See Press Release, U.S. Atty’s Office for the Southern Dist. of TX, First-ever False Claims Act settlement received from Paycheck Protection Program lender (Sept. 13, 2022), https://www.justice.gov/usao-sdtx/pr/first-ever-false-claims-act-settlement-received-paycheck-protection-program-lender.
[22] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Pharmaceutical Company Akorn Agrees to Pay $7.9 Million for Allegedly Causing Medicare to Pay for Invalid Prescription Drugs (Sept. 15, 2022), https://www.justice.gov/opa/pr/pharmaceutical-company-akorn-agrees-pay-79-million-allegedly-causing-medicare-pay-invalid.
[23] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Biogen Inc. Agrees to Pay $900 Million to Settle Allegations Related to Improper Physician Payments (Sept. 26, 2022), https://www.justice.gov/opa/pr/biogen-inc-agrees-pay-900-million-settle-allegations-related-improper-physician-payments; Stacy Cowley, Biogen Agrees to Pay $900 Million to Settle Lawsuit Over Kickbacks, N.Y. Times (Sept. 26, 2022), https://www.nytimes.com/2022/09/26/business/biogen-900-million-lawsuit-kickbacks.html.
[24] See Press Release, U.S. Atty’s Office for the Northern Dist. of GA, DermaTran and three other pharmacies to pay over $6.8 million to settle civil claims (Oct. 17, 2022), https://www.justice.gov/usao-ndga/pr/dermatran-and-three-other-pharmacies-pay-over-68-million-settle-civil-claims.
[25] See Press Release, U.S. Atty’s Office for the Northern Dist. of CA, Sutter Health Agrees to Pay $13 Million to Settle False Claims Act Allegations of Improper Billing for Lab Tests (Oct. 17, 2022), https://www.justice.gov/usao-ndca/pr/sutter-health-agrees-pay-13-million-settle-false-claims-act-allegations-improper.
[26] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Carter Healthcare Affiliates and Two Senior Managers to Pay $7.175 Million to Resolve False Claims Act Allegations for False Florida Home Health Billings (Oct. 18, 2022), https://www.justice.gov/opa/pr/carter-healthcare-affiliates-and-two-senior-managers-pay-7175-million-resolve-false-claims#:~:text=October%2018%2C%202022-,Carter%20Healthcare%20Affiliates%20and%20Two%20Senior%20Managers%20to%20Pay%20%247.175,False%20Florida%20Home%20Health%20Billings
[27] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Modernizing Medicine Agrees to Pay $45 Million to Resolve Allegations of Accepting and Paying Illegal Kickbacks and Causing False Claims (Nov. 1, 2022), https://www.justice.gov/opa/pr/modernizing-medicine-agrees-pay-45-million-resolve-allegations-accepting-and-paying-illegal.
[28] See Press Release, U.S. Atty’s Office for the Western Dist. of TX, Omega Healthcare Investors, Inc. Agrees to Pay $3 Million to Settle Civil False Claims Act Allegations (Nov. 9, 2022), https://www.justice.gov/usao-wdtx/pr/omega-healthcare-investors-inc-agrees-pay-3-million-settle-civil-false-claims-act.
[29] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Florida Birth-Related Neurological Injury Compensation Plan and Association to Pay $51 Million to Resolve False Claims Act Allegations (Nov. 14, 2022), https://www.justice.gov/opa/pr/florida-birth-related-neurological-injury-compensation-plan-and-association-pay-51-million.
[30] See Press Release, U.S. Atty’s Office for the Dist. of NJ, Opioid Abuse Treatment Facility to Pay $3.15 Million for Kickback Violations, Obstructing Federal Audit, and False Claims Submitted to Government Insurance Programs (Dec. 5, 2022), https://www.justice.gov/usao-nj/pr/opioid-abuse-treatment-facility-pay-315-million-kickback-violations-obstructing-federal.
[31] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Three Health Care Providers Agree to Pay $22.5 Million for Alleged False Claims to California’s Medcaid Program (Dec. 7, 2022), https://www.justice.gov/opa/pr/three-health-care-providers-agree-pay-225-million-alleged-false-claims-california-s-medicaid.
[32] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Ocenture LLC and Careluina LLC Settle Allegations of False Claims for Unnecessary Genetic Testing (Dec. 15, 2022), https://www.justice.gov/opa/pr/ocenture-llc-and-carelumina-llc-settle-allegations-false-claims-unnecessary-genetic-testing.
[33] See U.S. Dep’t of Health & Hum. Servs., Centers for Medicare & Medicaid Servs., Medicare Program; Contract Year 2024 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, Medicare Parts A, B, C, and D Overpayment Provisions of the Affordable Care Act and Programs of All-Inclusive Care for the Elderly; Health Information Technology Standards and Implementation Specifications, 87 FR 79452 (Dec. 14, 2022), https://www.govinfo.gov/content/pkg/FR-2022-12-27/pdf/2022-26956.pdf (hereinafter “Overpayment Proposed Rule”).
[34] E.g., 42 C.F.R. § 401.305(a)(2).
[35] See 42 U.S.C. § 1320a-7k(d); 31 U.S.C. § 3729(a)(1)(G).
[36] See UnitedHealthcare Ins. Co. v. Azar, 330 F. Supp. 3d 173, 191 (D.D.C. 2018), rev’d in part on other grounds sub nom. UnitedHealthcare Ins. Co. v. Becerra, 16 F.4th 867 (D.C. Cir. 2021), cert. denied, 142 S. Ct. 2851 (U.S. June 21, 2022).
[37] See Overpayment Proposed Rule, 87 FR 79452, at 79559.
[38] See U.S. Dep’t of Health & Hum. Servs., Centers for Medicare & Medicaid Servs., Medicare and Medicaid Programs; Policy and Technical Changes to the Medicare Advantage, Medicare Prescription Drug Benefit, Program of All-inclusive Care for the Elderly (PACE), Medicaid Fee-For-Service, and Medicaid Managed Care Programs for Years 2020 and 2021, –– FR –– (Jan. 30, 2023), https://public-inspection.federalregister.gov/2023-01942.pdf.
[39] See id. at 1.
[40] Id. at 2.
[41] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Justice Department Announces COVID-19 Fraud Strike Force Teams (Sept. 14, 2022), https://www.justice.gov/opa/pr/justice-department-announces-covid-19-fraud-strike-force-teams.
[42] Id.
[43] See Office of Inspector General, U.S. Dep’t of Health & Human Servs., Medicare Telehealth Services During the First Year of the Pandemic: Program Integrity Risks (Sept. 2022), https://oig.hhs.gov/oei/reports/OEI-02-20-00720.pdf.
[44] Office of Inspector General, U.S. Dep’t of Health & Hum. Servs., Labs with Questionably High Billing for Additional Tests Alongside COVID‑19 Tests Warrant Further Scrutiny (Dec. 2022), https://oig.hhs.gov/oei/reports/OEI-09-20-00510.pdf.
[45] See id. at 4–10.
[46] See Press Release, U.S. Atty’s Office for the Southern Dist. of TX, First-ever False Claims Act settlement received from Paycheck Protection Program lender (Sept. 13, 2022), https://www.justice.gov/usao-sdtx/pr/first-ever-false-claims-act-settlement-received-paycheck-protection-program-lender.
[47] See Gibson, Dunn & Crutcher LLP, 2021 Year-End False Claims Act Update (Feb. 3, 2022), https://www.gibsondunn.com/2021-year-end-false-claims-act-update/#_ednref55.
[48] Congressional Budget Office, Cost Estimate: S. 2428, False Claims Amendments Act of 2021 (July 15, 2022), https://www.cbo.gov/system/files?file=2022-07/s2428.pdf.
[49] See PPP and Bank Fraud Enforcement Harmonization Act of 2022, Pub. L. 117-166, 136 Stat. 1365, https://www.congress.gov/117/plaws/publ166/PLAW-117publ166.pdf; COVID-19 EIDL Fraud Statute of Limitations Act of 2022, Pub. L. 117-165, 136 Stat. 1363, https://www.congress.gov/117/plaws/publ165/PLAW-117publ165.pdf.
[50] See Gibson, Dunn & Crutcher LLP, Emergency Federal Measures to Combat Coronavirus (Mar. 18, 2020), https://www.gibsondunn.com/emergency-federal-measures-to-combat-coronavirus/.
[51] See Press Release, House Comm. on Small Business, Chairwoman Velázquez, Ranking Member Luetkemeyer Introduces Bills to Extend Statute of Limitations on COVID Small Business Fraud Cases (Apr. 1, 2022), https://smallbusiness.house.gov/news/documentsingle.aspx?DocumentID=404060.
[52] See HHS-OIG, State False Claims Act Reviews, https://oig.hhs.gov/fraud/state-false-claims-act-reviews/ (last visited Jan. 9, 2023) (FCA Reviews); 42 U.S.C. § 1396h(a).
[53] 42 U.S.C. § 1396h(b)(2).
[54] Id. § 1396h(b)(3).
[55] FCA Reviews, supra n.52.
[56] 42 U.S.C. § 1320a-7b(b).
The following Gibson Dunn lawyers assisted in the preparation of this alert: Jonathan Phillips, Winston Chan, John Partridge, James Zelenay, Reid Rector, Michael Dziuban, Chelsea Knudson, Blair Watler, John Turquet Bravard, Ben Gibson, Julien Jabari, Wynne Leahy, Jose Madrid, Nick Perry, Kelsey Stimson, Adrienne Tarver, and Chumma Tum.
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues. Please feel free to contact the Gibson Dunn lawyer with whom you usually work, the authors, or any of the following members of the firm’s False Claims Act/Qui Tam Defense Group:
Washington, D.C.
Jonathan M. Phillips – Co-Chair, False Claims Act/Qui Tam Defense Group (+1 202-887-3546, jphillips@gibsondunn.com)
F. Joseph Warin (+1 202-887-3609, fwarin@gibsondunn.com)
Joseph D. West (+1 202-955-8658, jwest@gibsondunn.com)
Geoffrey M. Sigler (+1 202-887-3752, gsigler@gibsondunn.com)
Lindsay M. Paulin (+1 202-887-3701, lpaulin@gibsondunn.com)
San Francisco
Winston Y. Chan – Co-Chair, False Claims Act/Qui Tam Defense Group (+1 415-393-8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415-393-8391, cstevens@gibsondunn.com)
New York
Reed Brodsky (+1 212-351-5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212-351-3850, mdenerstein@gibsondunn.com)
Alexander H. Southwell (+1 212-351-3981, asouthwell@gibsondunn.com)
Brendan Stewart (+1 212-351-6393, bstewart@gibsondunn.com)
Denver
John D.W. Partridge (+1 303-298-5931, jpartridge@gibsondunn.com)
Robert C. Blume (+1 303-298-5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303-298-5784, mloseman@gibsondunn.com)
Ryan T. Bergsieker (+1 303-298-5774, rbergsieker@gibsondunn.com)
Reid Rector (+1 303-298-5923, rrector@gibsondunn.com)
Dallas
Robert C. Walters (+1 214-698-3114, rwalters@gibsondunn.com)
Andrew LeGrand (+1 214-698-3405, alegrand@gibsondunn.com)
Los Angeles
Nicola T. Hanna (+1 213-229-7269, nhanna@gibsondunn.com)
Timothy J. Hatch (+1 213-229-7368, thatch@gibsondunn.com)
Deborah L. Stein (+1 213-229-7164, dstein@gibsondunn.com)
James L. Zelenay Jr. (+1 213-229-7449, jzelenay@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650-849-5395, bwagner@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP. All rights reserved. For contact and other information, please visit us at www.gibsondunn.com.
Attorney Advertising: These materials were prepared for general informational purposes only based on information available at the time of publication and are not intended as, do not constitute, and should not be relied upon as, legal advice or a legal opinion on any specific facts or circumstances. Gibson Dunn (and its affiliates, attorneys, and employees) shall not have any liability in connection with any use of these materials. The sharing of these materials does not establish an attorney-client relationship with the recipient and should not be relied upon as an alternative for advice from qualified counsel. Please note that facts and circumstances may vary, and prior results do not guarantee a similar outcome.
Hundreds of millions of dollars in government recoupments. Supreme Court attention. Potential Congressional legislation. All of this—and more—marked the False Claims Act (FCA) landscape during the first half of 2022, and proved yet again that the FCA is one of the government’s most powerful, and most litigated, enforcement tools.
On the enforcement front, the U.S. Department of Justice (DOJ) announced FCA resolutions totaling more than $500 million during the first half of the year, outpacing last year’s settlements. Among those resolutions were settlements related to fraud under the COVID stimulus programs and novel settlements from DOJ’s nascent “cyber-fraud” initiative, which promises to blur the line between traditional cybersecurity law and traditional FCA claims. DOJ also settled its usual assortment of cases against health care companies and government contractors.
Meanwhile, the Supreme Court agreed to decide yet another FCA case—this time to decide how much control the government retains over FCA litigation pursued by whistleblowers on its behalf—marking the 10th time in the last 15 years that the Supreme Court has decided to clarify aspects of the FCA statutory framework. The Supreme Court’s grant of certiorari adds to a host of important circuit court decisions from the last six months, as well as continued rumblings about potential Congressional action to strengthen the FCA.
With all of these developments, Gibson Dunn is pleased to once again present our mid-year round-up of the critical developments that businesses and practitioners must know about under the FCA.
Below, we summarize recent enforcement activity, then provide an overview of notable legislative and policy developments at the federal and state levels, and finally analyze significant court decisions from the past six months. Gibson Dunn’s recent publications regarding the FCA may be found on our website, including in-depth discussions of the FCA’s framework and operation, industry-specific presentations, and practical guidance to help companies navigate the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
I. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE FIRST HALF OF 2022
During the first half of 2022, DOJ announced FCA resolutions totaling more than $500 million. Enforcement activity thus far in 2022 has outpaced that of the first six months of 2021, although many of the resolutions announced this year have been relatively modest in amount. It remains to be seen whether DOJ will match the recoveries obtained during 2021, which included blockbuster settlements stemming from the opioid crisis.
Some of the most notable settlements of the first six months of 2022 came from the continued fallout from COVID and a new DOJ initiative around cyber-fraud.
Specifically, DOJ continues to focus on enforcement actions related to the Paycheck Protection Program (PPP), including actions under the FCA. For example, as detailed below, in February DOJ reached a settlement with a Virginia-based software development company to resolve allegations that the company fraudulently obtained multiple PPP loans in the year 2020.[1] In April, DOJ announced a settlement with a medical provider network, and several individuals, of claims that the company billed for unnecessary telehealth visits and instructed physicians to order certain medical tests without assessing for medical necessity.[2] In addition, DOJ claimed that the company submitted false statements in connection with a PPP loan application, by representing in its PPP loan application that the company was not engaged in unlawful activity. Notably, all four of the qui tam actions resolved by the settlement pre-dated the creation of the PPP program under the CARES Act. In addition to FCA claims, the settlement resolved a claim under the Financial Institutions Reform, Recovery and Enforcement Act (FIRREA), ostensibly based entirely on the PPP allegations. The addition of this claim follows the approach taken in DOJ’s very first PPP-related settlement in January 2021, which we have covered previously.
DOJ also continues to focus on employing the FCA to respond to cybersecurity threats. In March, DOJ announced its first settlement under the “Cyber Fraud Initiative” that it announced late last year.[3] The Cyber Fraud Initiative was set up to encourage the federal government to pursue fraud claims related to cybersecurity, including claims related to data security practices by health care providers. In this particular settlement, a Florida-based medical services provider agreed to pay $930,000 to resolve allegations that it failed to disclose to the State Department that it had not consistently stored patients’ medical records in a secure electronic medical record system and that it failed to properly obtain certain controlled substances that were manufactured in accordance with federal quality standards. Given the significance of data security in the health care industry, it is not surprising that DOJ’s first settlement under the Cyber Fraud Initiative was with a medical company—but we expect to see DOJ extending the initiative’s efforts broadly across industries in the coming months. Indeed, on July 8, DOJ announced another first-of-its-kind FCA settlement with a defense and aerospace contractor who allegedly misrepresented its compliance with cybersecurity requirements in certain federal government contracts.
Below, we summarize the other most notable settlements from the first half of the year, organized by industry and focused on key theories of liability at issue in the resolutions. As is often the case, FCA recoveries in the health care and life sciences industries dominated enforcement activity during the first half of the year in terms of the number and value of settlements. DOJ, however, also announced notable resolutions in the government contracting and procurement space, described below.
A. Health Care and Life Science Industries
Settlements to resolve liability under the FCA in the health care and life sciences industries totaled more than $400 million in the first half of 2022.
- On January 11, a California health system agreed to pay $3 million to resolve allegations that it violated the FCA by ordering and submitting referrals for unnecessary genetic testing, leading to the submission of false claims to Medicare for those tests.[4]
- On January 12, a specialized footwear company agreed to pay $5.5 million to settle allegations that the company sold shoe inserts to diabetic patients who received a prescription for the inserts from a physician. According to the government, the company billed Medicare and Medicaid as if the inserts provided to patients were customized, but the inserts actually all came from a generic model. In connection with the settlement, the company entered a three-year corporate integrity agreement, requiring the company to update its policies and hire an independent review organization to monitor the company’s Medicare and Medicaid claims. The settlement also resolved a qui tam suit brought by a former employee; that individual’s share of the recovery was not reported with the settlement announcement.[5]
- On January 31, a health care company agreed to pay more than $13 million to settle allegations that it violated the FCA and AKS by providing initial discounts on the purchase of drugs to physician practices. The government alleged the company used these discounts to persuade the practices to buy federally reimbursable drugs from the company rather than its competitors. According to the government, the company’s upfront discounts ran afoul of the FCA and AKS because they were not tied to any particular sale and were not associated with an earned rebate. The settlement also resolved qui tam suits brought against the health care company, for which the relators received approximately $2.8 million of the settlement.[6]
- On February 9, a hospital agreed to pay $3.8 million to resolve allegations that it violated the FCA and AKS by paying its own cardiologists to cover for, and provide services to, another cardiologist’s patients when that cardiologist was unavailable. The government alleged that the cardiologist, in turn, referred millions of dollars of medical procedures to the hospital. The government asserts the arrangement constituted an unlawful kickback and resulted in the submission of false claims to the government. The settlement also resolved a qui tam suit brought by a former hospital employee; that individual’s share of the recovery was not reported with the settlement announcement.[7]
- On February 15, three Ohio-based health care providers agreed to pay more than $3 million to resolve allegations that the providers submitted bills to Medicare for complex surgeries by an orthopedic surgeon who worked out of each of the providers’ facilities. The government asserted that the surgeon claimed to have performed numerous procedures that he actually did not perform. Even though none of the parties knowingly submitted false claims based on the surgeons’ actions, the government alleged there was sufficient evidence that each provider should have known that the claims were false.[8]
- On March 7, a pharmaceutical company agreed to pay $260 million to resolve allegations that it violated the FCA and AKS by underpaying Medicaid rebates for a particular drug and using a foundation to subsidize patient co-pays. Approximately $234.7 million of the settlement went to resolving the rebate allegations and $26.3 million went toward resolving the kickback claims. In addition to the payment, the pharmaceutical company agreed to a corporate integrity agreement, which includes monitoring provisions focused on Medicaid rebates. The settlement also resolved qui tam suits brought by two whistleblowers, who received almost $30 million of the settlement.[9]
- On March 28, a psychiatrist agreed to pay $3 million to resolve allegations that he violated the FCA by billing the Department of Labor Office of Worker’s Compensation Programs for psychiatric appointments that never took place and double-billing for other sessions. As part of the settlement, the psychiatrist agreed to be excluded from federal health care programs for 25 years.[10]
- On April 6, a health care system and four affiliated entities agreed to pay $20 million to resolve allegations that they violated the FCA by making donations to a local entity which in turn contributed the money to the state’s Medicaid program—the state ultimately paid back the funds to the health care system. The government asserted that, based on this conduct, the government had to make matching Medicaid payments without any actual expenditure by the state. The settlement also resolved a qui tam suit brought by a former hospital reimbursement manager, who received $5 million of the settlement.[11]
- On April 12, a pain management company agreed to pay $24.5 million to resolve allegations that it violated the FCA by submitting claims for unnecessary drug, genetic, and psychological testing. As part of the settlement, the company entered into a corporate integrity agreement with the Office of Inspector General for the Department of Health and Human Services (HHS-OIG) that required the company to maintain a compliance department and submit to ongoing reviews by an independent review organization. The settlement resolved qui tam suits brought by former employees of the company and its affiliates; the relators’ share of the recovery was not reported with the settlement announcement.[12]
- On April 13, a company, its co-founders, and 18 affiliated anesthesia entities agreed to pay $7.2 million to resolve allegations that they violated the FCA and AKS by sharing revenue received from the company’s anesthesia services with the physicians running outpatient surgery centers in order to obtain exclusive anesthesia agreements with the surgery centers. The settlement also resolved a qui tam suit brought by a whistleblower, who received $1.3 million of the settlement.[13]
- On April 29, a hearing aid company agreed to pay $34.4 million to resolve allegations that it violated the FCA by submitting inaccurate claims for reimbursements to the federal government. Some Federal Employees Health Benefits Program plans elect to offer a benefit for hearing aids but require submission of a hearing-loss related diagnosis code supported by a hearing exam by a physician. The government alleged that the company submitted claims for hearing aids containing unsupported diagnosis codes to the Benefits Program.[14]
- On May 9, a home health company agreed to pay $2.1 million to resolve allegations that it violated the FCA by submitting claims for Medicare beneficiaries who were not homebound and did not require certain skilled care. The government also alleged the company submitted claims for services that otherwise did not have a valid or appropriate plan of care and/or did not have requisite in-person encounters to qualify for home health service certification. The settlement resolved allegations brought in a qui tam and a HHS-OIG complaint; the whistleblower’s share of the recovery was not disclosed at the time of the settlement.[15]
- On June 1, a behavioral health care provider agreed to pay $2.1 million to settle claims that it improperly billed claims to Medicaid that were ineligible for reimbursement under the state’s medical clinical coverage policy. The allegations stemmed from a qui tam lawsuit; the whistleblower’s share was not disclosed at the time of the settlement announcement.[16]
- On June 1, a molecular science company agreed to pay over $2.8 million to resolve allegations that it billed Medicare for laboratory tests in violation of Medicare’s 14-Day Rule, which prohibits laboratories from separately billing for certain tests ordered within 14 days of a patient’s discharge from an inpatient or outpatient hospital setting. In addition to submitting purportedly improper claims, the government alleged that the company failed to discourage providers who ordered testing within 14 days after a discharge from canceling the order and placing a new order for testing after the 14-day period had elapsed. The settlement partially resolves one qui tam lawsuit and fully resolves another.[17]
- On June 6, a diagnostics company that provides home sleep testing agreed to pay $3.5 million to resolve FCA and AKS allegations that it billed Medicare and four other federal health care companies for unnecessary home sleep testing. The government alleged that the company’s founder directed employees to submit claims for additional nights of home sleep testing when only one night was necessary to effectively diagnose sleep apnea. The government further alleged that the company improperly multiplied copays received from Medicare beneficiaries and incentivized physicians to refer all home sleep testing services to the company. The settlement agreement requires the company’s founder and vice president to pay $300,000 and $125,000, respectively, and the company and its founder agreed to a corporate integrity agreement. The allegations in the settlement were part of two qui tam [18]
- On June 10, a Los Angeles doctor agreed to pay $9.5 million to resolve FCA allegations that he submitted claims to Medicare for procedures and tests that he never performed and admitted that he intentionally submitted false claims for payment. The settlement amount includes nearly $5.5 million paid as criminal restitution following a guilty plea to health care fraud in a separate criminal matter. The allegations originally stemmed from a qui tam lawsuit filed by a former medical assistant and former IT consultant. The two whistleblowers will receive more than $1.75 million as their share of the recovery.[19]
- On June 21, a managed care health services company and its previously-owned subsidiary agreed to pay $4.6 million to resolve allegations that it billed a joint federal and state Medicaid program for care provided by unlicensed and unsupervised staff. The settlement also resolved allegations that the companies failed to provide and timely document the provision of adequate clinical supervision for clinicians. The settlement resolves a qui tam suit filed by four former employees; the whistleblowers were awarded $810,000 as their share of the recovery.[20]
B. Government Contracting and Procurement
Settlements to resolve liability under the FCA in the government contracting and procurement space totaled more than $90 million in the first half of 2022.
- On February 23, a kitchen and food service equipment company agreed to pay $48.5 million to resolve allegations that it provided inaccurate information to the government regarding contracts awarded to small businesses. The federal government may set aside certain contracts for various categories of small businesses; and, in some instances, only eligible small businesses may bid on and receive contracts. The government alleged that the company caused federal agencies to award contracts to small businesses that claimed to be run by service-disabled veterans when, in reality, the small businesses served as the face of the contracts, and the company actually provided all of the services. The settlement resolved a qui tam suit brought by a competing company, which received $10.9 million of the settlement.[21]
- On March 7, a construction contractor agreed to pay $10 million to resolve allegations it overbilled the government. The government asserted that the contractor—which was performing work for the Department of Energy—presented false invoices for non-existent materials submitted by a subcontractor to the contractor. According to the government, the contractor’s employees received kickbacks from the subcontractor to submit the claims.[22]
- On March 14, two freight carrier companies agreed to pay $6.9 million to resolve allegations they violated the FCA by inflating bills submitted to the Department of Defense. The government alleged the companies each claimed to have hauled greater weights than they actually carried, which served as the basis for payment under the contract. The settlement resolved a qui tam suit brought by a former employee of one of the companies who received $1.3 million of the settlement.[23]
- On March 21, a package delivery company agreed to pay $5.3 million to resolve allegations that it violated the FCA by submitting inaccurate information regarding time and proof of delivery. Under the company’s contract for mail pick-up and delivery at various Department of Defense and State Department locations domestically and abroad, the company received penalties for mail delivered late or to the wrong location. The government alleged that the company submitted scans of proof of mail and package deliveries that did not accurately reflect when the company actually delivered the packages.[24]
- On May 12, a construction company agreed to pay $2.8 million to settle FCA allegations that the company improperly manipulated a subcontract reserved for service-disabled, veteran-owned small businesses (SDVOSBs). The government awarded the company a contract to develop retirement communities and residential facilities for veterans, a condition of which was to provide subcontracting opportunities to SDVOSBs. The company admitted that it negotiated with a non-SDVOSB for the subcontract and then entered into a subcontract with an SDVOSB for the same work, but with an additional 1.5% fee. The company further admitted that it should have known the SDVOSB was a pass-through for the non-SDVOSB, which provided all of the work under the subcontract. The settlement resolves allegations originally brought in a qui tam lawsuit; the whistleblower received approximately $630,000 for its share of the recovery.[25]
- On May 18, seven South Korean companies agreed to pay $3.1 million to settle FCA and other allegations that they conspired to rig the bidding process for contracts for construction and engineering work on United States military bases in South Korea. The government alleged that, as a result of the anticompetitive behavior, the government paid more for services performed under the contracts than it otherwise would have.[26]
- On May 25, a manufacturing company agreed to pay $3 million to settle allegations that it violated the FCA by knowingly selling technical fabrics to the military that failed to meet required specifications. The company allegedly falsified test results and falsely certified that its military-grade fabrics met all requisite performance specifications set by the military. The company also entered into an agreement with the Defense Logistics Agency to ensure that it remains in compliance with testing requirements going forward. The settlement resolves allegations brought under a qui tam lawsuit; the whistleblower’s share was not disclosed at the time of the settlement announcement.[27]
- On June 2, a manufacturing company and two related entities agreed to pay $5.2 million to resolve allegations that the company violated the FCA by improperly obtaining a contract reserved for small businesses that it was ineligible to receive. The manufacturing company allegedly falsely certified that it was a “small business concern” within the meaning of the Small Business Administration’s regulations so as to receive 22 small business set-aside contracts, even though the company ceased to qualify after its acquisition by a larger company. The company also allegedly falsely certified that it was a “women-owned small business concern.” As part of the settlement agreement, the entities received credit for the company’s voluntary disclosure and cooperation with the government during the investigation.[28]
- On June 14, four companies agreed to pay $13.7 million to resolve FCA and AKS allegations that the companies rigged the bidding process for subcontracts to perform logistics support services for the military in Iraq and that employees entered into arrangements with a foreign contractor under which the companies would receive a kickback for every subcontract awarded to the foreign entity. The government alleged that the employees influenced the federal government to award two subcontracts to the foreign contractor at prices higher than necessary to fulfill the military’s contract requirements, and the government alleged that the companies extended the duration of subcontracts at inflated prices and sought reimbursement of these inflated costs from the U.S. military. The settlement resolves allegations originally brought in a qui tam lawsuit; the whistleblower’s share was not disclosed at the time of the settlement announcement.[29]
C. Other
Settlements to resolve other types of FCA cases totaled nearly $25 million in the first half of 2022.
- On January 14, a loan servicer agreed to pay $7.9 million to resolve allegations that it violated the FCA by submitting inaccurate claims to the Department of Education. The government alleged that the loan service failed to make required financial adjustments to borrower accounts and improperly treated some ineligible borrowers as eligible for military deferments.[30]
- On April 4, a telecommunications carrier agreed to pay $13.4 million to resolve allegations that it enrolled 175,000 ineligible customers for free cell phones and service under a federal program. The federal government runs the Lifeline Program, which assists low-income individuals with telecommunications needs. According to the government, the carrier failed to monitor subscriptions obtained by a third-party marketing firm who actually enrolled the ineligible customers. The settlement also resolved a qui tam suit brought by a former employee of the marketing firm, who received roughly $450,000 of the settlement.[31]
- On May 27, a for-profit school and its owner agreed to pay over $1 million to settle allegations that they improperly concealed financial information to influence the school’s student loan default rate, which affects an institution’s ability to participate in Title IV programs. The for-profit school and its owner allegedly mailed 154 direct payments to loan servicers on behalf of 102 students to prevent those students from defaulting on their loans and, therefore, counting towards the school’s student loan default rate. The school and its owner allegedly failed to disclose the actual student loan default rate to the Department of Education. The school and its founder also entered into an administrative agreement with the Department of Education.[32]
II. LEGISLATIVE AND POLICY DEVELOPMENTS
A. Federal Legislative Developments
As we previously reported, last summer, Senator Chuck Grassley (R-IA), along with a bipartisan group of Senators, introduced a bill to amend the FCA which he subsequently amended last November. Senator Grassley’s proposed amendments were targeted at limiting the implications of the Supreme Court’s decision in Escobar and limiting the government’s ability to dismiss claims brought by relators. Since being reported out of the Senate Judiciary Committee, there has been no indication regarding whether the bill will receive a floor vote.
Time will tell whether the Supreme Court’s decision to take up the Polansky case (which relates to the government’s ability to dismiss claims brought by relators, as covered below in this Alert) has the effect of further delaying or killing the bill’s progress. In the meantime, Senator Grassley filed an amicus curiae brief in support of a certiorari petition in the United States ex rel. Schutte v. SuperValue Inc., which deals with the relevance of a defendant’s subjective beliefs for FCA scienter.[33] Consistent with his statements in the past, Sen. Grassley’s brief focuses on what he sees as the importance of a defendant’s contemporaneous subjective intent, in a professed effort to prevent the same defendant’s “post-hoc” (albeit objectively correct) interpretations of the law from hobbling the government’s efforts to establish scienter.
B. State Legislative Developments
The first half of 2022 has witnessed significant developments in state-level FCA legislation. Most notably, Colorado expanded its false claims law beyond the realm of Medicaid fraud. The Colorado False Claims Act (CFCA), which became law on June 7, 2022, largely tracks the federal FCA, but with several significant features not found in the federal statute.
First, the CFCA expressly states that “[a] person who acts merely negligently with respect to information is not deemed to have acted knowingly, unless the person acts with reckless disregard of the truth or falsity of the information.”[34] The federal FCA contains neither an express carve-out for negligence (although courts routinely find that it does not satisfy the Act’s scienter requirement), nor any sort of caveat regarding situations in which negligence could still be actionable.
Second, the CFCA contains a distinct framework for assessing reduced damages and penalties for cooperating defendants. The federal FCA grants courts discretion to impose only double damages when a defendant reports information within 30 days of obtaining it, cooperates fully with the government, and discloses the information prior to the commencement of any action under the FCA and without actual knowledge of any FCA investigation. The CFCA, by contrast, requires the imposition of double damages for any defendant who reports information within 30 days of learning it, does so without actual knowledge of the existence of an FCA investigation, and does so while an FCA action is under seal.[35] In the event that a similarly situated defendant reports the information prior to any action being filed under seal, the court is required to impose one-and-one-half the amount of damages.[36] In this way, the CFCA places a premium on companies enhancing their compliance programs to affirmatively identify fraudulent conduct, but arguably incentivizes qui tam relators to act hastily in filing complaints in an effort to lock even cooperating defendants into at least double damages. On another level, the apparently mandatory nature of the reduced damages provisions in cases where defendants make voluntarily self-disclosures could have the effect of making settlement discussions in such cases more efficient by vesting the government with less discretion to negotiate damages multipliers where the other requirements for cooperation credit are otherwise met.
Third, and notably in light of Polansky and the longer history of disputes at the federal level regarding DOJ’s dismissal authority, the CFCA explicitly requires the Colorado Attorney General to consider certain enumerated factors when determining whether to voluntarily dismiss a CFCA action.[37] Those factors are “the severity of the false claim, program or population impacted by the false claim, duration of the fraud, weight and materiality of the evidence, other means to make the program whole, and other factors that the Attorney General deems relevant.”[38] The statute also expressly provides that “[t]he Attorney General’s decision-making process concerning a motion to dismiss and any records related to the decision‑making process are not discoverable in any action.”[39]
Fourth, unlike the federal FCA, the CFCA expressly prohibits a qui tam relator from disclosing—as part of its mandatory disclosure statement served on the State along with a copy of the complaint—”any evidence or information that the person reasonably believes is protected by the defendant’s attorney-client privilege unless the privilege was waived, inadvertently or otherwise, by the person who holds the privilege; an exception to the privilege applies; or disclosure of the information is permitted by an attorney pursuant to [the SEC’s standards of professional conduct], the applicable Colorado Rules of Professional Conduct, or otherwise.”[40]
Elsewhere, other states have been actively considering steps to expand or revise their false claims laws. In Connecticut and Michigan, bills are pending that would—like Colorado’s new law—expand false claims liability beyond Medicaid, although without nearly as much variation on matters of FCA procedure and practice compared to the federal statute as is reflected in the CFCA.[41] New York’s legislature, for its part, on June 3 passed an amendment to the state’s FCA that would expand liability for tax-related claims to include fraudulent failures to file tax returns. As currently written, the New York FCA covers tax-related actions but limits them to the knowing use of false records and statements material to tax obligations.[42] The new bill is now awaiting the governor’s signature.
HHS-OIG provides incentives for states to enact false claims statutes in keeping with the federal FCA. HHS-OIG approval for a state’s FCA confers an increase of 10 percentage points in that state’s share of any recoveries in cases involving Medicaid.[43] Such approval requires, among other things, that the state FCA in question “contain provisions that are at least as effective in rewarding and facilitating qui tam actions for false or fraudulent claims” as are the federal FCA’s provisions.[44] Approval also requires a 60-day sealing provision and civil penalties that match those available under the federal FCA.[45] Consistent with our reporting in prior alerts, the lists of “approved” and “not approved” state statutes remain at 22 and 7, respectively.[46] Michigan is on the “not approved” list, and could remain there even if its FCA amendment passes: the bill entitles qui tam relators to a maximum of 20% of recoveries in intervened cases, whereas the federal FCA caps that amount at 25%.[47] HHS-OIG could well determine that this discrepancy means the Michigan law (if it passes in its current form) is not “at least as effective” as the federal FCA is in rewarding qui tam relators.
III. CASE LAW DEVELOPMENTS
The big news of the last six months was the Supreme Court’s decision to wade into the FCA waters once more. But the first half of 2022 also saw a number of notable federal appellate court decisions. We cover all of these developments below.
A. Supreme Court and Multiple Courts of Appeal Consider DOJ’s Dismissal Authority
1. Supreme Court Grant of Cert
The Supreme Court granted certiorari in United States ex rel. Polansky v. Executive Health Resources, Inc., 17 F.4th 376, 385 (3d Cir. 2021), cert. granted, 142 S. Ct. 2834 (2022), to decide the question of whether the government can dismiss a qui tam realtor lawsuit after declining to litigate, and if it can, what the government must show in order to persuade the district court to dismiss the case. 21-1052, United States, Ex Rel. Polansky v. Executive Health Resources, Inc., https://www.supremecourt.gov/qp/21-01052qp.pdf (last visited July 14, 2022).
The FCA generally provides that the government may dismiss a qui tam, over the objection of a relator, at any time, subject to certain procedures. 31 U.S.C. § 3730. This provision is an important check on runaway whistleblower suits, United States ex rel. Campos v. Johns Hopkins Health Sys. Corp., 2018 WL 1932680, at *8 (D. Md. April 24, 2018), and is a critical feature that courts have relied upon to uphold the constitutionality of the qui tam provisions against constitutional challenges under the delegation clause, United States ex rel. Stilwell v. Hughes Helicopters, Inc., 714 F.Supp. 1084, 1086–93 (C.D. Cal. 1989).
Currently, however, there is a circuit split as to the standard under which a district court may evaluate the government’s decision to dismiss relators’ cases.[48] Some courts have concluded that the government may dismiss virtually any action brought on behalf of the government, with very little scrutiny. Polansky, 17 F.4th at 384–88. Other courts have decided that if the government does not intervene in a relator’s case, the government must first intervene in the lawsuit before seeking to dismiss it under Federal Rule of Civil Procedure 41(a)’s standard. Id. Still other courts have indicated that the government must have some reasonable basis for the decision to dismiss, and ostensibly apply a degree of scrutiny to dismissal decisions. Id.
In Polansky, appellant Jesse Polansky argues that the Supreme Court must adjudicate the “intractable split” on the issue, urging the Court to hold the government to a heightened standard. Id., Pet. at I. Unsurprisingly, respondent Executive Health Resources—seeking to preserve DOJ’s decision to dismiss—contends that the standards are just “slightly different” and that appellant would lose under all of them. Id., Opp. at 1.
FCA practitioners know that the “split” may be more of an illusion than a reality. In practice, district courts almost always agree to dismiss cases where DOJ seeks dismissal, regardless of what jurisdiction they are in and what standard they apply. Indeed, in every Circuit Court case making up the split, the court upheld the government’s dismissal. It is therefore unclear why the Supreme Court decided to hear the case, given the lack of practical differences in the standards. But we will be watching carefully to see whether the Supreme Court strengthens—or weakens—DOJ’s ability to reign-in qui tam lawsuits.
2. First and Eleventh Circuits Consider the Government’s Dismissal Authority
While the Supreme Court’s grant of cert in Polansky was the big news with regard to the government’s dismissal authority, several circuit courts also issues decisions that bear on DOJ’s control over qui tams.
The FCA provides for a hearing when the Government moves to dismiss a relator’s qui tam action over the relator’s objection. But the statute is silent as to the standards governing that hearing and the courts of appeals have developed different tests for assessing the propriety of such a motion to dismiss. Weighing in on the issue for the first time, the First Circuit held in Borzilleri v. Bayer Healthcare Pharmaceuticals, Inc., 24 F.4th 32 (1st Cir. 2022), that the Government must “always provide its reasons for seeking dismissal” and that the “court’s role is to apply commonly recognized principles for assessing government conduct—the well-established ‘background constraints on executive action.’” Id. at 42. The motion to dismiss should be granted unless the relator can establish that the government’s decision to seek dismissal “transgresses constitutional limitations” or that the government “is perpetrating a fraud on the court.” Id. Further, if the relator seeks discovery to establish the government’s “improprieties” the relator must make a “substantial threshold showing” to support her claims. Id. at 44.
In so holding, the First Circuit, disagreed with the approaches taken by other circuits. For example, the Borzilleri Court held that the Ninth Circuit’s approach, which requires the government to identify a “valid government purpose” for dismissal and to establish a “rational relation between dismissal and accomplishment of the purpose” erred in placing too weighty a burden on the government. Id. at 37, 40 (quoting United States ex rel. Sequoia Orange Co. v. Baird-Neece Packing Corp., 151 F.3d 1139, 1145 (9th Cir. 1998)). The Borzilleri Court likewise rejected the approach taken by the Seventh and Third Circuits, which look to Federal Rule of Civil Procedure 41 for guidance. The First Circuit concluded that Rule 41 was not an “appropriate guide” for the FCA because its primary aim is to protect the defendant from being prejudiced by a plaintiff’s voluntary dismissal, while § 3730(c)(2)(A) hearings are intended to protect the relator’s “unique” interests as an “objecting co-plaintiff.” Id. at 41.
In United States ex rel. Farmer v. Republic of Honduras, 21 F.4th 1353 (11th Cir. 2021), meanwhile, the Eleventh Circuit took up the issue of whether the Government must formally intervene in a qui tam action to move for dismissal, where it has initially declined to intervene. Id. at 1355. There, when relators filed their initial complaint in the qui tam action, the United States declined to intervene. Id. Later, however, after the relators filed an amended complaint adding defendants, the United States—without first filing a motion to intervene in the case—motioned to dismiss the action. Id. The relators challenged the dismissal motion on the ground that the Government was not a party to the suit because it had not formally intervened “for good cause” under 31 U.S.C. § 3730(c)(3), and thereby lacked standing to motion for dismissal. Section 3730 asserts that Courts may allow the Government to later intervene—in a case for which it initially declined intervention—upon a showing of “good cause.” Id. at § 3730(c)(3). However, the Court held that the Government was not required to show “good cause” for late interventions that strictly seek dismissal, explaining that the good-cause subsection “applies only when the [G]overnment intervenes for the purpose of actually proceeding with the litigation,” rather than intervening “for the purpose of settling and ending the case.” Id. at 1356. “[W]hen the Government moves to dismiss an action after having declined to intervene,” the Court continued, “it need provide the Relator only notice and a hearing.” Id. at 1357. Notably, the Eleventh Circuit subsequently voted to rehear the case en banc, and accordingly vacated the initial panel’s opinion.
B. Public Disclosure Bar and First-to-File
The FCA employs two related rules barring relators from bringing actions in situations where the underlying, alleged wrongdoing has already been disclosed or addressed by someone else. First, the public disclosure bar requires dismissal of FCA cases brought by private litigants where “substantially the same allegations or transactions” underlying the action have already been publicly disclosed, including “in a Federal criminal, civil, or administrative hearing in which the Government or its agent is a party,” unless the relator is an “original source of the information.” 31 U.S.C. § 3730(e)(4). Relatedly, the first-to-file rule prevents private litigants from bringing an action that is “based on the facts underlying” any other action that was pending at the time and brought by a separate litigant. Id. at § 3730(b)(5). There were a number of notable decisions under these bars in the first half of the year.
1. Eleventh Circuit Considers Public Disclosure and First-to-File Bars
In Cho on behalf of States v. Surgery Partners, Inc., 30 F.4th 1035 (11th Cir. 2022), the Eleventh Circuit considered questions related to both the first-to-file rule and the public disclosure bar.
First, it considered whether an amended complaint filed after a related action was resolved can overcome the first-to-file rule’s applicability to an earlier compliant that was filed while a related case was still pending. Id. at 1038, 1040. In April 2017, relators filed a qui tam action against a private equity firm and its subsidiary for allegedly leading a fraudulent enterprise to submit false claims for reimbursement under Medicare. Id. at 1037, 1039. However, in August 2016, approximately six months before the relators filed their complaint, a different group of relators had filed a related action against one of the same parties—the subsidiary—but not against the parent, private equity firm. Id. at 1039. After the August 2016 action settled and became public, the relators for the April 2017 action filed an amended complaint, which focused the allegations solely on the private equity firm that had not been a party in the separate, but related, action brought by the different relators in the August 2016 action. Id. However, the district court dismissed the second amended complaint, finding that though the amended complaint was filed after the August 2016 case was resolved, the first-to-file rule rendered the entire action dismissible because the initial, April 2017 complaint was filed when the August 2016 suit was still pending. Id.
Under de novo review, the Eleventh Circuit affirmed the district court’s dismissal under the first-to-file rule on appeal. Focusing on the key words “bring” and “action” within section 3730(b)(5) (establishing that “no person . . . may intervene to bring a related action based on the facts underlying [a] pending action”), the Court asserted that the first-to-file rule “turns on the moment the Relators initiated legal proceedings.” Id. at 1040 (emphasis in original). The Court accordingly concluded that the plain text of the FCA “tethers” the first-to-file analysis on the “moment a qui tam action is filed.” Id. at 1042.
The Eleventh Circuit also considered a nuance to the public disclosure bar: what test it should apply when determining whether a pending action is “related” to a later-filed qui tam action. Id. Adopting the same approach used by its sister circuits and the district court below, the Court decided to use the “same material elements” test to assess relatedness. Id. Under this test, two actions are deemed to be related if they “rely on the same ‘essential facts.’” Id. (quoting United States ex rel. Wood v. Allergan, Inc., 899 F.3d 163, 169 (2d Cir. 2018)). Applying this test to the facts at hand, the Court found that the relators’ April 2017 action was adequately “related” to the separate relators’ August 2016 action for the purposes of dismissal, explaining that though the April 2017 complaint named an additional defendant not included in the August 2016 action, the suits were related because the first-to-file bar does not “require[] a necessarily defendant-specific approach[,] . . . particularly where the new defendant is a corporate relative or affiliate of the earlier-named defendants.” Id. at 1043.
2. Ninth Circuit Considers What Counts as a Public Disclosure
In a pair of cases, the Ninth Circuit considered what public disclosures can trigger the public disclosure bar.
First, the Ninth Circuit addressed whether materials released by a government agency under FOIA can trigger the public disclosure bar in Roe v. Stanford Health Care, No. 20-55874, 2022 WL 796798 (9th Cir. Mar. 15, 2022). In that case, appellant brought a FCA suit alleging that Stanford Health Care engaged in fraudulent Medicare billing. The Ninth Circuit affirmed the district court’s dismissal of appellant’s claims on the basis of the FCA’s public disclosure bar. Appellant’s claims were barred because the “second amended complaint is almost entirely premised on publicly disclosed Medicare data [appellant] obtained through Freedom of Information Act requests,” and because “[t]he other information [appellant] identifies . . . is either irrelevant or already revealed in the data.” Id., at *1. In so holding, the Ninth Circuit joined the vast majority of courts to consider the issue in holding that FOIA disclosures do trigger the public disclosure bar.
Second, in Mark ex rel. United States v. Shamir USA, Inc., No. 20-56280, 2022 WL 327475 (9th Cir. Feb. 3, 2022), the Ninth Circuit considered whether an eyeglass lens manufacturer’s description of its customer rewards program in public promotional materials triggered the FCA’s public disclosure bar. The qui tam relator in this case alleged that Shamir’s customer rewards program violated the AKS and FCA by exploiting the Government’s practice of reimbursing lenses based on the invoice price. Id. at *1. According to the relator, Shamir persuaded eyecare professionals (ECPs) to prescribe Shamir’s lenses by offering discounts and rebates on lenses and subsequently providing the ECPs with invoices purporting to charge full price so that government insurance programs, “rather than Shamir, pa[id] for the ECP discounts.” Id. The district court granted Shamir’s motion to dismiss the relator’s claim, holding that his allegations were precluded by the FCA’s public disclosure bar because they were “substantially similar” to statements Shamir made about its rewards program in promotional materials. Id. For example, in several industry journals, Shamir encouraged ECPs to participate in its rewards program by stating that “they automatically receive rewards back, making it a win-win for everyone,” and offering to develop “personalized YouTube channels” for ECPs to showcase Shamir-manufactured lenses. Id. at *2. According to the district court, these “publicly disclosed facts” announced that the discounts and rebates ECPs received from Shamir “were not deducted from any insurance reimbursement,” thereby foreclosing the relator’s claim. Id. The Ninth Circuit overruled the district court, holding that application of the public disclosure bar was not warranted because the information in the promotional materials “was so innocuous” that no “transaction or allegation of fraud” was publicly disclosed by Shamir in the first place. Id.
C. Sixth Circuit Finds Inflated Fixed-Price Proposals Sufficient to Satisfy FCA’s Pleading Standard
In United States ex rel. USN4U, LLC v. Wolf Creek Federal Services, Inc., 34 F.4th 507 (6th Cir. 2022), the Sixth Circuit issued a detailed and probing decision that addressed pleading standards for FCA suits. In that case, the relator, USN4U, LLC (USN4U) alleged that Wolf Creek Federal Services, Inc. (Wolf Creek), a federal contractor, “falsely inflated project estimates to the National Aeronautics and Space Administration (NASA) for facilities maintenance projects to be performed by Wolf Creek, resulting in the negotiation of fraudulently induced, exorbitant contract prices,” thereby violating the FCA. Id. at 510.
Wolf Creek provided facilities management maintenance services to the National Aeronautics and Space Administration (NASA) under the terms of an indefinite-delivery indefinite-quantity (IDIQ) contract awarded in 2013 (the NASA Contract). Id. at 510–11. Pursuant to the terms of the NASA Contract, NASA would approve specific projects for Wolf Creek to perform on a firm-fixed price basis. Id. at 511. After Wolf Creek received a work order for the subject task, it was required to submit a proposal for schedule of completion and the total cost of labor and materials, which NASA would evaluate for purposes of negotiating a final firm-fixed price amount. Id. As the Court noted, once the firm-fixed price was established, Wolf Creek’s invoices were required to align with the agreed-upon amount. Id.
Wolf Creek filed a motion to dismiss USN4U’s complaint for failure to state a valid claim, taking the position that “the estimates and project proposals [it submitted] were not ‘claims’ for FCA purposes” and generally contesting the sufficiency of USN4U’s fraud claims more generally. Id. at 512. After USN4U amended its complaint, Wolf Creek filed a second motion to dismiss, “repeating their argument that quotes were not ‘claims’ for purposes of the FCA and further arguing that invoices were not ‘false’ if they matched the quoted amount.” Id. The Court noted that USN4U’s amended complaint included further examples supporting the FCA allegations, including providing a list of employees who admitted to reporting more hours worked than actually completed, as well as “a transcript of a recorded conversation in which several Wolf Creek employees allegedly discussed the fraudulent scheme.” Id.
The district court nevertheless granted Wolf Creek’s second motion to dismiss and denied USN4U’s motion to file a second amended complaint. By the district court’s read, notwithstanding that the work order proposals Wolf Creek submitted to NASA contained quoted prices, the proposals did not constitute “claims” under the FCA, serving only as estimates rather than demands or invoices. Id. at 512–13. Additionally, the district court held that USN4U did not satisfy its burden to plead falsity under the FCA as USN4U’s allegations merely compared the labor costs with industry standards to support its claims of false inflation. Id. at 513. Finally, the district court held that USN4U failed to satisfy its burden to plead fraud in the inducement, citing Wolf Creek’s continued performance under the NASA Contract even after the fraud allegations materialized. Id.
The Sixth Circuit reversed, holding USN4U sufficiently alleged a claim of fraudulent inducement, which is a viable legal theory under the FCA, noting that “FCA liability can be based on a fraudulent premise that caused the United States to enter into a contract,” and finding that USN4U adequately pled its fraudulent inducement claim based on its assertions that “Wolf Creek falsely inflated cost estimates in its work order proposals and thus induced NASA to agree to contracts at that price point.” Id. (internal citation omitted).
Turning next to the elements of an FCA claim, the Court first addressed falsity, finding that reliance on industry standards as the basis for a fraud claim is not presumptively insufficient. See id. at 515. The Court also noted that USN4U provided additional support beyond a comparison with industry standards when it offered evidence of a disparity in billing activity between the employees participating in the scheme and those who did not, an incident where a plumber billed to a project where no plumbing work was required, and a recording transcript in which Wolf Creek employees discussed the practice of using false estimates. Id.
Regarding scienter, the Court found USN4U satisfied the pleading standard through, in addition to the examples discussed herein, USN4U’s submission of a “recorded conversation in which Wolf Creek employees allegedly discussed their knowledge of the falsely inflated cost estimates and labor hours,” noting that an employee stated: “[t]he original estimate that they gave me for hours, they told me they needed about 130 hours of overtime. I upped it like I always do to 164 hrs.” Id. at 516. The employee further stated:
I came back and we started chewing up what you guys had. It was going away so I got nervous and had no intentions of working 40 hrs when I came back. So then I got crazy and started pumping out estimates. And now it[‘]s, if I stay at the rate that I am at right now we will never run out. So the key is to just have it flooded. Inundate the customer with the quotes.
Id. With respect to materiality, the Court found that “Wolf Creek’s falsely inflated estimates could have had the tendency to influence NASA’s contracting decisions,” given that NASA relied on Wolf Creek’s contractual estimate rather than conduct its own research into costs. Id. The Court noted that “[w]hile it is possible, as Wolf Creek suggests, that NASA’s faith in Wolf Creek’s estimates came from its own careful research and consideration of Wolf Cree’s proposals, it is also plausible that NASA trusted and relied exclusively upon Wolf Creek’s estimates, and that NASA ultimately paid Wolf Creek based on its induced belief that the quoted prices were reasonably accurate.” Id. Finally, the Court stated that NASA’s decision to allow Wolf Creek to continue with contract performance after the fraud allegations surfaced was not dispositive or indicative of “actual knowledge” of fraud, and noted that various factors could influence the decision to continue performance, including the desire to avoid prematurely ending a contractual relationship prior to an investigation into the alleged fraud. Id. at 517. The court also noted that the government’s decision not to intervene in a particular case is not considered for purposes of assessing materiality. Id.
Lastly, the Court found that USN4U satisfied the pleading requirements for causation, stating that “NASA asked Wolf Creek for estimates and when it awarded Wolf Creek the contracts, NASA always awarded the contracts for the quoted amount, which could indicate that NASA trusted and relied upon the purported accuracy of Wolf Creek’s estimates when it entered into the contracts at the quoted prices.” Id. at 518. The Court also noted that “NASA plausibly would not have agreed to pay Wolf Creek the quoted amount if NASA knew that it was being grossly overcharged.” Id. The Court accordingly reversed the judgment of the district court and remanded for further proceedings.
D. Falsity
1. Ninth Circuit Holds Disagreement in Clinical Judgment Is Insufficient to Establish Falsity
In Holzner v. DaVita Inc., No. 21-55261, 2022 WL 726929 (9th Cir. Mar. 10, 2022), appellant alleged that DaVita Inc. (appellee) provided medically unnecessary products and services and/or unreasonably expensive medications in violation of the FCA.
The Ninth Circuit affirmed the district court’s dismissal of appellant’s claims on the grounds that appellant had not plausibly alleged a false statement in order to establish FCA liability. Id. at *2. The court explained that the complaint “does not contain sufficient facts . . . to state a plausible claim of false or fraudulent billing related to the appellees’ provision of dialysis treatments” and prescription drugs, because the allegations instead “show no more than a disagreement in clinical judgment,” as “[t]he medical literature on which Holzner relies . . . does not establish new guidelines for practitioners or otherwise compel a change of practice among nephrologists.” Id. at *1. As a result, “Holzner has not raised a plausible inference that the nephrologists’ certifications that these interventions are medically necessary—or appellees’ reliance on those certifications—were false or fraudulent.” Id.
In so holding, the Ninth Circuit joins a growing number of appeals courts to consider these issues in recent years. In United States v. AseraCare, Inc., 938 F.3d 1278 (11th Cir. 2019), the Eleventh Circuit similarly held that clinical disagreement is insufficient to establish falsity because the FCA requires the alleged falsehood to be objectively false. Yet the Third Circuit, in United States ex rel. Druding v. Care Alternatives, 952 F.3d 89 (3d Cir. 2020), and the Sixth Circuit, in United States v. Paulus, 894 F.3d 267 (6th Cir. 2018), have rejected the Eleventh and Ninth Circuits’ conclusion that the FCA requires proof of “objective falsity,” and held instead that a difference of medical opinion can be sufficient to show that a statement is false.
2. District Court Holds That Relator Failed to Satisfy Falsity Element of an FCA Claim Based on Alleged Failure to Comply with State Law
In United States ex rel. Jehl v. GGNSC Southaven LLC, 3:19-CV-091-NBB-JMV, 2022 WL 983644 (N.D. Miss. Mar. 30, 2022), the district court held, inter alia, that the relator failed to satisfy the falsity element of an FCA claim based on the Defendants’ alleged false certification of compliance with state licensure laws. In the complaint, the qui tam relator alleged that the Defendants, who operated a nursing facility in Southaven, Mississippi, violated the FCA by billing Medicare and Medicaid for health care services while certifying that the company complied with Mississippi’s licensure laws for nurses even though its Director of Nursing Services (Director) was not licensed to work as a nurse in the state. Id. at *1. Shortly before she began working for the defendants in Mississippi, the Director obtained a valid multistate nursing license from Virginia based in part on a declaration she submitted averring that Virginia was her primary state of residence (PSOR). Id. at *2. The Virginia multistate license permitted the Director to practice nursing in Mississippi, and the day after the Director began her employment at the Southaven facility, an employee for one of the Defendants confirmed that she held an active Virginia nursing license with a multistate privilege. Id. However, according to the relator, the Director’s multistate license was actually invalid because her claiming of Virginia as her PSOR was false; in fact, the relator continued, the Director’s PSOR was actually Tennessee, as evidenced by her Tennessee driver’s license. Id. The relator argued that because the Director lacked a valid license to practice nursing in Mississippi while employed by the Defendants, their “certifications of compliance with applicable licensure laws in their Medicare and Medicaid reimbursement requests were false within the meaning of the FCA.” Id.
The district court granted the Defendants’ motion for summary judgment, holding that the relator did not possess evidence establishing the FCA’s falsity, knowledge, or materiality elements. Id. at *6. The Centers for Medicare & Medicaid Services’ (CMS) regulations for nursing facilities require such facilities to comply “with all applicable Federal, State, and local laws, regulations, and codes.” 42 C.F.R. § 483.70(b). In CMS’s State Operations Manual, which provides interpretive guidance on CMS’s nursing facility regulations, CMS explains that noncompliance “with Federal, State, and local laws, regulations [and] codes” occurs “only when a final adverse action has been taken by the authority having jurisdiction regarding noncompliance with its applicable laws, regulations, codes and/or standards.” Id. at *4. In this case, undisputed facts showed that during the period when the Director worked at the Southaven facility, neither the Virginia Nursing Board nor any other nursing board had “taken any action, let alone a final adverse action, against [the Director’s] professional license, meaning that under CMS’s clear rules, her nursing license was . . . valid during the entire period of her employment.” Id. at *5. Therefore, as a matter of law, the FCA’s falsity element could not be satisfied because the Defendants’ certifications of compliance with CMS regulations were “demonstrably true and accurate, not false.” Id. at *6. Similarly, the district court concluded that the relator could not satisfy the knowledge element of an FCA claim because the Defendants’ certifications were proper. Id. Further, the district court ruled that the relator could not satisfy the FCA’s materiality element because the CMS regulations that the Defendants allegedly breached, 42 C.F.R. Part 483, contained only “broad certification language” that, under established precedent, cannot support an FCA claim, and the evidence available at summary judgment “show[ed] no linkage between nurse licensure” and government payment of submitted claims. Id.
E. Materiality
1. D.C. Circuit Holds That the FCA’s Materiality Inquiry Focuses on the Potential Effect of False Statement When Made
In United States ex rel. Vermont National Telephone Co. v. Northstar Wireless, LLC, et al., 34 F.4th 29, 31 (D.C. Cir. 2022), Vermont National Telephone Company (Vermont Telephone) alleged that several telecommunications companies, including Northstar, SNR, DISH, and affiliated companies (collectively, Defendants), violated the FCA and defrauded the U.S. government of $3.3 billion by manipulating Federal Communications Commission (FCC) rules and falsely certifying their eligibility for discounts on spectrum licenses. The district court dismissed Vermont Telephone’s qui tam suit, relying on the FCA’s “government-action bar” and the FCA’s “demanding materiality standard.” Id. The D.C. Circuit reversed on both grounds.
To apportion licenses allowing companies to use portions of the electromagnetic spectrum to provide television, cell phone, and wireless internet service, the FCC holds auctions that involve a two-step license application process. Id. at 31. The FCC officers allocate “bidding credits” (discounts to cover part of the cost of licenses won at auction) to very small businesses, those with less than $15 million in revenue. Id. at 31, 32. As part of the application process, companies must provide information concerning their eligibility to bid in the auction and certify their eligibility for bidding credits. Id. at 32.
Vermont Telephone alleged that Defendants failed to disclose resale agreements with DISH, which would have increased their attributable revenues beyond the allowable cap for the very small business credits. Id. at 36. Defendants argued that the alleged undisclosed agreements would not have changed the FCC’s ultimate decision to deny bidding credits because the FCC found the Defendants ineligible for the discounts even without disclosure of any resale agreements. Id. at 37.
The D.C. Circuit rejected Defendants’ argument to focus on the “ultimate decision.” Id. Instead, the Court’s materiality analysis focused on the “potential effect of the false statement when it is made,” not on “the false statement’s actual effect after it is discovered.” Id. (internal citation omitted). The Court held that Defendants’ failure to disclose agreements central to their eligibility for discounts was certainly “capable of influencing” the FCC’s eligibility determination and, thus, Vermont Telephone plausibly pleaded materiality. Id. at 36–38. This appears to conflict with language from Escobar that if the Government pays a particular claim in full despite its actual knowledge that certain requirements were violated then “that is very strong evidence” of the immateriality of those requirements. Universal Health Servs., Inc. ex rel. Escobar v. United States, 579 U.S. 176, 195 (2016).
2. Ninth Circuit Enforces False Certification and Materiality Pleading Requirements
In McElligott v. McKesson Corp., No. 21-15477, 2022 WL 728903, at *1 (9th Cir. Mar. 10, 2022), appellant relators alleged that McKesson “knowingly present[ed], or cause[d] to be presented, a false or fraudulent claim for payment or approval” by making false certifications in violation of the FCA. The Ninth Circuit affirmed the district court’s dismissal of relators’ claims without leave to amend because the complaint failed to plead a claim for express false certification, as there were no allegations that “defendant submitted a claim for payment to the government in which it expressly certified that it had complied with a specific law or provision of the contract with which it knew it had not complied.” Id.
Nor did the relators sufficiently allege that Defendant made implied false certifications. “[T]he second amended complaint does not allege that, in its claims for payment, McKesson made specific representations about the medical supplies it provided that were rendered misleading half-truths by its failure to disclose noncompliance with material statutory, regulatory, or contractual requirements.” Id. Instead, “[a]s far as the complaint reveals, McKesson represented nothing more in its claims for payment than that it delivered certain medical supplies on certain dates,” and “[t]he complaint does not allege that those representations were false.” Id.
The Court also ruled that the relators failed to allege materiality, as “nothing in the complaint gives rise to a reasonable inference that the security of McKesson’s supply chain was material to the government’s decision to pay for medical supplies that McKesson actually delivered.” Id. at *2.
F. Scienter
1. Fourth Circuit Struggles in Determining When a Defendant’s Alleged Mistakes of Law Can Establish Scienter
In two recent cases, Fourth Circuit panels divided as to whether a defendant’s alleged misinterpretation of a complex regulation could establish scienter under the FCA.
In the first case, United States ex rel. Sheldon v. Allergan Sales, LLC, 24 F.4th 340 (4th Cir. 2022), Judge Wilkinson, joined by Judge Richardson, affirmed the district court’s dismissal of an FDCA case and imported the scienter standard from the Supreme Court’s Fair Credit Reporting Act decision in Safeco Ins. Co. of America v. Burr, 551 U.S. 57 (2007), into the FCA context. Safeco “set forth a two-step analysis” in determining whether a defendant has acted in reckless disregard of the law. Sheldon, 24 F.4th at 347. First, a court asks “whether defendant’s interpretation was objectively reasonable.” Id. The second step is “determining whether authoritative guidance might have warned defendant away from that reading.” Id. This test is appropriate in FCA cases, reasoned the majority, because the “FCA defines ‘knowingly’ as including actual knowledge, deliberate ignorance, and reckless disregard. . . . [and] Safeco interpreted ‘willfully’ to include both knowledge and recklessness.” Id. at 348.
The court then applied Safeco to the facts. This case concerned the Medicaid Drug Rebate Statute, which requires “manufacturers seeking to have their drugs covered by Medicaid [to] enter into Rebate Agreements with the Secretary of Health and Human Services and provide quarterly rebates to states on Medicaid sales of covered drugs. . . . For covered drugs, the rebate amount is the greater of two numbers: (1) the statutory minimum rebate percentage, or (2) the difference between the Average Manufacturer Price and the Best Price,” the latter of which is essentially “the lowest price available from the manufacture.” Id. at 345.
Plaintiff employee filed a qui tam suit against Forest Laboratories, LLC under the FCA, alleging that Forest gave discounts to customers but failed to account for these discounts in calculating Best Price, resulting in false reports to the government. Id. at 343–44. Forest argued that it correctly, or at least reasonably, interpreted the meaning of “Best Price” and therefore did not knowingly defraud the government.
The majority agreed. Pursuant to the Safeco standard, “[u]nder the FCA, a defendant cannot act ‘knowingly’ if it bases its actions on an objectively reasonable interpretation of the relevant statute when it has not been warned away from that interpretation by authoritative guidance. This objective standard precludes inquiry into a defendant’s subjective intent.” Id. at 348. Forest did not “act knowingly under the FCA” because “Forest’s reading of the Rebate Statute was at the very least objectively reasonable and because it was not warned away from that reading by authoritative guidance.” Id. at 343–44, 347.
Judge Wynn dissented. He accused the majority of “effectively neuter[ing] the False Claims Act . . . by eliminating … two of its three scienter standards (actual knowledge and deliberate ignorance) and replacing the remaining standard with a test (objective recklessness) that only the dimmest of fraudsters could fail to take advantage of.” Id. at 357 (Wynn, J., dissenting). Judge Wynn would not have “imported” Safeco into the FCA, a “vastly different statutory context.” Id. at 361. The Fourth Circuit subsequently granted rehearing en banc, 2022 WL 1467710, but did not vacate the panel opinion.
The second case, United States ex rel. Gugenheim v. Meridian Senior Living, LLC, 36 F.4th 173 (4th Cir. 2022), concerned reimbursement for “personal care services,” including assisting with activities such as eating, dressing, and bathing, that are provided to elderly or disabled adults under North Carolina’s Medicaid program. The program authorizes a certain number of daily “personal care services” for elderly or disabled patients based on a patient’s personal needs. Id. at 175–76. Defendant adult-care homes billed for the authorized hours of personal care services rather than the actual amount of services provided. Id. at 177–78.
Plaintiff attorney filed a qui tam suit against the nursing homes under the FCA, alleging that the homes’ billing schemes violated the rules of the state Medicaid program. The district court granted summary judgment to the home, holding that the plaintiff failed to show that the home’s claims “were materially false or made with the requisite scienter.” Id. at 178.
A divided panel of the Fourth Circuit affirmed. At issue was whether the defendants knowingly submitted false claims to Medicaid. Judge Rushing, joined by Judge Wilkinson, concluded that the defendants did not. Id. at 175. They emphasized that state regulations defining billing for personal care services were unclear and that the defendants plausibly interpreted the regulations as allowing their billing practices. They then held that courts cannot infer scienter when defendants reasonably interpret ambiguous regulations:
We need not determine whether Defendants’ interpretation of [state regulations] is correct. The policy and related guidance from NC Medicaid are sufficiently ambiguous to foreclose the possibility of proving scienter based solely on the clarity of the regulation. We cannot infer scienter from an alleged regulatory violation itself, and we especially will not do so where there is regulatory ambiguity as to whether Defendants’ conduct even violated the policy.
Id. at 181 (quotation marks removed). The court then rejected plaintiff’s alternate argument that the home should “have sought more guidance about an ambiguous regulation” because there was no evidence that the home “knew, or even suspected, that [its] interpretation of [the regulation] was incorrect.” Id. Plaintiff failed to submit “any evidence that Defendants knew, or even suspected, that their interpretation of [the regulation] and the related guidance from NC Medicaid was incorrect (indeed, it may be right).” Id.
Senior Judge Traxler dissented and would have allowed the case to proceed to trial. The plaintiff submitted plausible evidence of overbilling and “that Defendants did next to nothing to educate themselves” about the regulation. Id. at 183 (Traxler, J., dissenting). Thus, “a reasonable jury could find that Defendants failed to make a reasonable and prudent inquiry into how [the regulation] affected their billing method and, instead, buried their heads in the sand to maximize their billings.” Id. at 190.
2. Fifth Circuit Reiterates Need to Allege Scienter
In United States ex rel. Jacobs v. Walgreen Company, 2022 WL 613160 (5th Cir. March 2, 2022), plaintiff pharmacist filed a qui tam suit against her employer Walgreens under the FCA, alleging that Walgreens submitted false claims for reimbursement to Medicare and Medicaid. The district court dismissed the case for failure to plead fraud with particularity. The Fifth Circuit affirmed in a short opinion.
The court began by describing the pleading requirements of the Act. A plaintiff must plead: “(1) a false statement or fraudulent course of conduct; (2) that was made or carried out with the requisite scienter; (3) that was material; and (4) that caused the government to pay out money (i.e., that involved a claim).” Id. at *1. But the plaintiff did not “plead[] facts supporting an inference that the allegedly fraudulent conduct amounted to anything more than innocent mistake or neglect.” Id. The complaint accordingly failed to state a claim because the FCA does not confer liability “for innocent mistakes or neglect.” Id. Indeed, the allegation that “Walgreens failed to correct certain billing mistakes once it discovered them” was an impermissibly “conclusory allegation[] that [did] not provide specifics as to the ‘who, what, when, where, and how of the alleged fraud.’” Id.
3. Seventh Circuit Reaffirms Objective Scienter Standard
In United States ex rel. Proctor v. Safeway, Inc., the relator alleged that between 2006 and 2015, Safeway knowingly submitted false claims to government health programs when it reported its “retail” price for certain drugs as its “usual and customary” price, even though many customers paid much less than the retail price due to discount programs. 30 F.4th 649, 652–54 (7th Cir. 2022). The allegations were almost identical to the allegations in United States ex rel. Schutte v. SuperValu, Inc., 9 F.4th 455 (7th Cir. 2021), which we covered in our 2021 Year End False Claims Act Update.
The Seventh Circuit decided SuperValu while Safeway was pending. Safeway, 30 F.4th at 657. In SuperValu, the Seventh Circuit held that the Supreme Court’s decision in Safeco Ins. Co. of America v. Burr, 551 U.S. 47 (2007) applied to the FCA’s scienter provision, meaning that a defendant does not act with “reckless disregard” as long as (1) its interpretation of the relevant statute or regulation is “objectively reasonable” and (2) no “authoritative guidance” warned it away from that interpretation. Id.
The court reached the same conclusion in Safeway, and further explained when guidance is “authoritative.” Id. at 660. In order for guidance to be “authoritative,” it must “come from a source with authority to interpret the relevant text.” Id. In addition to the source, the Seventh Circuit also considers whether that guidance was sufficiently specific to put a defendant on notice that its conduct is unlawful. Id. Accordingly, the court held that a single footnote in a lengthy manual that can be revised at any time is not authoritative guidance. Id. at 663
G. Sixth Circuit Holds that the Limitations Period for FCA Claims Begins to Run When Retaliation Occurs, Not When Relator Receives Notice
The Sixth Circuit recently reaffirmed that there is “no notice requirement” in the FCA statute of limitations for retaliation claims. El-Khalil v. Oakwood Healthcare, Inc., 23 F.4th 633 (6th Cir. 2022). The statute sets forth a three-year limitations period that begins to run when “the retaliation occurred.” Id. at 635 (quoting 31 U.S.C. § 3730(h)). The Court noted this conclusion is “hardly groundbreaking,” it merely codifies the “standard rule” that the “limitation period begins when the plaintiff ‘can file suit and obtain relief.’” Id. The El-Khalil Court did note, however, that equitable doctrines may toll the limitations period if an employer purposely delays its provision of notice in order to let the limitations period run and deprive the relator of a fair opportunity to bring suit. Id. at 636.
IV. CONCLUSION
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2022 False Claims Act Year-End Update, which we will publish in January 2023.
____________________________
[1] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Northern Virginia Company Settles False Claims Act Allegations of Improper Paycheck Protection Program Loan (Feb. 11, 2022), https://www.justice.gov/opa/pr/northern-virginia-company-settles-false-claims-act-allegations-improper-paycheck-protection.
[2] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Physician Partners of America to Pay $24.5 Million to Settle Allegations of Unnecessary Testing, Improper Remuneration to Physicians and a False Statement in Connection with COVID-19 Relief Funds (April 12, 2022), https://www.justice.gov/opa/pr/physician-partners-america-pay-245-million-settle-allegations-unnecessary-testing-improper.
[3] See Press Release, U.S. Atty’s Office for the Eastern Dist. of NY, Contractor Pays $930,000 to Settle False Claims Act Allegations Relating to Medical Services Contracts at State Department and Air Force Facilities in Iraq and Afghanistan (March 8, 2022), https://www.justice.gov/usao-edny/pr/contractor-pays-930000-settle-false-claims-act-allegations-relating-medical-services.
[4] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, UC San Diego Health Pays $2.98 Million to Resolve Allegations of Ordering Unnecessary Genetic Testing (Jan. 11, 2022), https://www.justice.gov/opa/pr/uc-san-diego-health-pays-298-million-resolve-allegations-ordering-unnecessary-genetic-testing.
[5] See Press Release, U.S. Atty’s Office for the Southern Dist. of FL, Diabetic Shoe Company Agrees to Pay $5.5 Million to Resolve False Claims Act Allegations Regarding “Custom” Shoe Inserts (Jan. 12, 2022), https://www.justice.gov/usao-sdfl/pr/diabetic-shoe-company-agrees-pay-55-million-resolve-false-claims-act-allegations.
[6] See Press Release, U.S. Atty’s Office for the Dist. of MA, Cardinal Health Agrees to Pay More than $13 Million to Resolve Allegations that it Paid Kickbacks to Physicians (Jan. 31, 2022), https://www.justice.gov/usao-ma/pr/cardinal-health-agrees-pay-more-13-million-resolve-allegations-it-paid-kickbacks.
[7] See Press Release, U.S. Atty’s Office for the Dist. of NH, Catholic Medical Center Agrees to Pay $3.8 Million to Resolve Kickback-Related False Claims Act Allegations (Feb. 9, 2022), https://www.justice.gov/usao-nh/pr/catholic-medical-center-agrees-pay-38-million-resolve-kickback-related-false-claims-act.
[8] See Press Release, U.S. Atty’s Office for the Southern Dist. of OH, 3 Central Ohio health providers to pay more than $3 million for improper claims submitted to Medicare and Ohio Bureau of Workers’ Compensation (Feb. 15, 2022), https://www.justice.gov/usao-sdoh/pr/3-central-ohio-health-providers-pay-more-3-million-improper-claims-submitted-medicare-0.
[9] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, Mallinckrodt Agrees to Pay $260 Million to Settle Lawsuits Alleging Underpayments of Medicaid Drug Rebates and Payment of Illegal Kickbacks (Mar. 7, 2022), https://www.justice.gov/opa/pr/mallinckrodt-agrees-pay-260-million-settle-lawsuits-alleging-underpayments-medicaid-drug.
[10] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, Philadelphia Psychiatrist to Pay $3 Million to Resolve Allegations of False Workers’ Compensation Claims (Mar. 28, 2022), https://www.justice.gov/usao-edpa/pr/philadelphia-psychiatrist-pay-3-million-resolve-allegations-false-workers-compensation.
[11] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, Florida’s BayCare Health System and Hospital Affiliates Agree to Pay $20 Million to Settle False Claims Act Allegations Relating to Impermissible Medicaid Donations (Apr. 6, 2022), https://www.justice.gov/opa/pr/florida-s-baycare-health-system-and-hospital-affiliates-agree-pay-20-million-settle-false.
[12] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, Physician Partners of America to Pay $24.5 Million to Settle Allegations of Unnecessary Testing, Improper Remuneration to Physicians and a False Statement in Connection with COVID-19 Relief Funds (Apr. 12, 2022), https://www.justice.gov/opa/pr/physician-partners-america-pay-245-million-settle-allegations-unnecessary-testing-improper.
[13] See Press Release, U.S. Atty’s Office for the Northern Dist. of GA, Paul D. Weir, John R. Morgan, M.D., Care Plus Management, LLC, and Anesthesia Entities pay $7.2 million to Resolve Kickback and False Claims Act Allegations (Apr. 13, 2022), https://www.justice.gov/usao-ndga/pr/paul-d-weir-john-r-morgan-md-care-plus-management-llc-and-anesthesia-entities-pay-72.
[14] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, Hearing Aid Company Eargo Inc. Agrees to Pay $34.37 Million to Settle Common Law and False Claims Act Allegations for Unsupported Diagnosis Codes (Apr. 29, 2022), https://www.justice.gov/opa/pr/hearing-aid-company-eargo-inc-agrees-pay-3437-million-settle-common-law-and-false-claims-act.
[15] See Press Release, U.S. Atty’s Office for the Southern Dist. of FL, Home Health Company Operating in Florida Pays $2.1 Million to Resolve False Claims Allegations (May 9, 2022), https://www.justice.gov/usao-sdfl/pr/home-health-company-operating-florida-pays-21-million-resolve-false-claims-allegations.
[16] See Press Release, U.S. Atty’s Office for the Western Dist. of NC, Healthkeeperz, Inc. To Pay $2.1 Million To Resolve False Claims Act Allegations (June 1, 2022), https://www.justice.gov/usao-wdnc/pr/healthkeeperz-inc-pay-21-million-resolve-false-claims-act-allegations.
[17] See Press Release, U.S. Atty’s Office for the Eastern Dist. of NY, Caris Life Sciences Pays over $2.8 Million to Settle False Claims Act Allegations from Delay in Submission of Genetic Cancer Screening Tests (June 1, 2022), https://www.justice.gov/usao-edny/pr/caris-life-sciences-pays-over-28-million-settle-false-claims-act-allegations-delay.
[18] See Press Release, U.S. Atty’s Office for the Northern Dist. of IL, Suburban Chicago Home Sleep Testing Company To Pay $3.5 Million To Settle Federal Health Care Fraud Suit (June 6, 2022), https://www.justice.gov/usao-ndil/pr/suburban-chicago-home-sleep-testing-company-pay-35-million-settle-federal-health-care#:~:text=CHICAGO%20%E2%80%94%20A%20suburban%20Chicago%20diagnostics,and%20unnecessary%20home%20sleep%20testing.
[19] See Press Release, Los Angeles Doctor to Pay $9.5 Million to Resolve Allegations of Fraud Against Medicare and Medi-Cal (June 10, 2022), https://www.justice.gov/usao-edca/pr/los-angeles-doctor-pay-95-million-resolve-allegations-fraud-against-medicare-and-medi.
[20] See Press Release, U.S. Atty’s Office for the Dist. of MA, Molina Healthcare Agrees to Pay Over $4.5 Million to Resolve Allegations of False Claims Act Violations (June 21, 2022), https://www.justice.gov/usao-ma/pr/molina-healthcare-agrees-pay-over-45-million-resolve-allegations-false-claims-act#:~:text=Molina%20Healthcare%20Agrees%20to%20Pay,Department%20of%20Justice.
[21] See Press Release, U.S. Atty’s Office for the Northern Dist. of NY, Government Contractor Agrees to Pay Record $48.5 Million to Resolve Claims Related to Fraudulent Procurement of Small Business Contracts Intended for Service-Disabled Veterans (Feb. 23, 2022), https://www.justice.gov/usao-ndny/pr/government-contractor-agrees-pay-record-485-million-resolve-claims-related-fraudulent.
[22] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, MOX Services Agrees to Pay $10 Million to Resolve Allegations of Knowingly Presenting False Claims to Department of Energy for Non-Existent Construction Materials (Mar. 7, 2022), https://www.justice.gov/opa/pr/mox-services-agrees-pay-10-million-resolve-allegations-knowingly-presenting-false-claims.
[23] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, Freight Carriers Agree to Pay $6.85 Million to Resolve Allegations of Knowingly Presenting False Claims to the Department of Defense (Mar. 14, 2022), https://www.justice.gov/opa/pr/freight-carriers-agree-pay-685-million-resolve-allegations-knowingly-presenting-false-claims.
[24] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, UPS to Pay $5.3 Million to Settle False Claims Act Allegations for Falsely Reporting Delivery Times of U.S. Mail Carried Internationally (Mar. 21, 2022), https://www.justice.gov/opa/pr/ups-pay-53-million-settle-false-claims-act-allegations-falsely-reporting-delivery-times-us-0.
[25] See Press Release, U.S. Atty’s Office for the Northern Dist. of NY, Construction Company Agrees to Pay $2.8 Million to Resolve Allegations of Small Business Subcontracting Fraud (May 12, 2022), https://www.justice.gov/usao-ndny/pr/construction-company-agrees-pay-28-million-resolve-allegations-small-business.
[26] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, Seven South Korean Companies Agree to Pay Approximately $3.1 Million to Settle Civil False Claims Act Allegations for Bid Rigging on U.S. Department of Defense Contracts (May 18, 2022), https://www.justice.gov/opa/pr/seven-south-korean-companies-agree-pay-approximately-31-million-settle-civil-false-claims-act.
[27] See Press Release, U.S. Atty’s Office for the Dist. of WV, United States Attorney Chris Kavanaugh Announces $3,000,000 Settlement in False Claims Act Case Against HEYtex USA (May 25, 2022), https://www.justice.gov/usao-wdva/pr/united-states-attorney-chris-kavanaugh-announces-3000000-settlement-false-claims-act.
[28] See Press Release, U.S. Atty’s Office for the Dist. of CT, Connecticut Companies Pay $5.2 Million to Resolve Allegations of False Claims Act Violations Concerning Fraudulently Obtained Small Business Contracts (June 2, 2022), https://www.justice.gov/usao-ct/pr/connecticut-companies-pay-52-million-resolve-allegations-false-claims-act-violations.
[29] See Press Release, Office of Public Affairs, U.S. Dep’t of Justice, KBR Defendants Agree to Settle Kickback and False Claims Allegations (June 14, 2022), https://www.justice.gov/opa/pr/kbr-defendants-agree-settle-kickback-and-false-claims-allegations.
[30] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Loan Servicer Agrees to Pay Nearly $8 Million to Resolve Alleged False Claims in Connection with Federal Education Loans (Jan. 14, 2022), https://www.justice.gov/opa/pr/loan-servicer-agrees-pay-nearly-8-million-resolve-alleged-false-claims-connection-federal.
[31] See Press Release, U.S. Atty’s Office for the Middle Dist. of FL, TracFone Wireless to Pay $13.4 Million to Settle False Claims Relating to FCC’s Lifeline Program (Apr. 4, 2022), https://www.justice.gov/usao-mdfl/pr/tracfone-wireless-pay-134-million-settle-false-claims-relating-fcc-s-lifeline-program.
[32] See Press Release, U.S. Atty’s Office for the Dist. of CT, School and Owner Pay Over $1 Million to Resolve Allegations of Attempts to Improperly Influence the School’s Student Loan Default Rate (May 27, 2022), https://www.justice.gov/usao-ct/pr/school-and-owner-pay-over-1-million-resolve-allegations-attempts-improperly-influence.
[33] Brief for Amicus Curiae Senator Charles E. Grassley In Support of Petitioners, United States ex rel. Tracy Schutte, et al. v. Supervalu Inc., et al., https://www.supremecourt.gov/DocketPDF/21/21-1326/225832/20220519154806836_21-1326%20Amicus%20Brief.pdf.
[34] Colorado False Claims Act, House Bill 22-1119, https://leg.colorado.gov/sites/default/files/2022a_1119_signed.pdf.
[35] Id.
[36] Id.
[37] Id. at § 24-31-1204(1)(b).
[38] Id.
[39] Id.
[40] Id.
[41] https://www.cga.ct.gov/2022/TOB/S/PDF/2022SB-00426-R02-SB.PDF; http://www.legislature.mi.gov/documents/2021-2022/billintroduced/House/htm/2022-HIB-6032.htm.
[42] See N.Y. State Fin. L. § 189(1)(g), (4)(a); https://legislation.nysenate.gov/pdf/bills/2021/S8815.
[43] See https://oig.hhs.gov/fraud/state-false-claims-act-reviews/; 42 U.S.C. § 1396h(a).
[44] Id.
[45] Id.
[46] Id.
[47] Compare http://www.legislature.mi.gov/documents/2021-2022/billintroduced/House/htm/2022-HIB-6032.htm with 31 U.S.C. § 3730(d)(1).
[48] See John Elwood, Dismissing False Claims Act cases, promoting prescription fentanyl, and a capital case, SCOTUSBLOG (June 7, 2022, 8:25 PM), https://www.scotusblog.com/2022/06/dismissing-false-claims-act-cases-promoting-prescription-fentanyl-and-a-capital-case/.
The following Gibson Dunn lawyers assisted in the preparation of this alert: Jonathan M. Phillips, Winston Y. Chan, John D.W. Partridge, James L. Zelenay Jr., Reid Rector, Chelsea B. Knudson, Allison Chapin, Michael R. Dziuban, Tessa Gellerson, Ben Gibson, Katie King, Nick Perry, Becca Smith, Chumma Tum, Mike M. Ulmer, Tim Velenchuk, Blair Watler, and Josh Zuckerman.
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues. Please feel free to contact the Gibson Dunn lawyer with whom you usually work, the authors, or any of the following members of the firm’s False Claims Act/Qui Tam Defense Group:
Washington, D.C.
Jonathan M. Phillips – Co-Chair, False Claims Act/Qui Tam Defense Group (+1 202-887-3546, jphillips@gibsondunn.com)
F. Joseph Warin (+1 202-887-3609, fwarin@gibsondunn.com)
Joseph D. West (+1 202-955-8658, jwest@gibsondunn.com)
Robert K. Hur (+1 202-887-3674, rhur@gibsondunn.com)
Geoffrey M. Sigler (+1 202-887-3752, gsigler@gibsondunn.com)
Lindsay M. Paulin (+1 202-887-3701, lpaulin@gibsondunn.com)
San Francisco
Winston Y. Chan – Co-Chair, False Claims Act/Qui Tam Defense Group (+1 415-393-8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415-393-8391, cstevens@gibsondunn.com)
New York
Reed Brodsky (+1 212-351-5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212-351-3850, mdenerstein@gibsondunn.com)
Alexander H. Southwell (+1 212-351-3981, asouthwell@gibsondunn.com)
Brendan Stewart (+1 212-351-6393, bstewart@gibsondunn.com)
Casey Kyung-Se Lee (+1 212-351-2419, clee@gibsondunn.com)
Denver
John D.W. Partridge (+1 303-298-5931, jpartridge@gibsondunn.com)
Robert C. Blume (+1 303-298-5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303-298-5784, mloseman@gibsondunn.com)
Ryan T. Bergsieker (+1 303-298-5774, rbergsieker@gibsondunn.com)
Reid Rector (+1 303-298-5923, rrector@gibsondunn.com)
Dallas
Robert C. Walters (+1 214-698-3114, rwalters@gibsondunn.com)
Andrew LeGrand (+1 214-698-3405, alegrand@gibsondunn.com)
Los Angeles
Nicola T. Hanna (+1 213-229-7269, nhanna@gibsondunn.com)
Timothy J. Hatch (+1 213-229-7368, thatch@gibsondunn.com)
Deborah L. Stein (+1 213-229-7164, dstein@gibsondunn.com)
James L. Zelenay Jr. (+1 213-229-7449, jzelenay@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650-849-5395, bwagner@gibsondunn.com)
© 2022 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice.
Thirty-five years ago, Congress ushered in the modern era of False Claims Act (“FCA”) enforcement when it enacted the False Claims Amendments Act of 1986. At the time, the FCA was a seldom-enforced statute that resulted in government recoveries each year counted in the tens of millions. Now, 35 years later, the FCA is firmly—and consistently from one administration to another—established as the government’s principal fraud enforcement tool, netting the government annual recoveries counted in the billions. This past year underscored the continuing impact of the FCA and attendant risks to companies that do business—directly or indirectly—with the government.
DOJ announced that it collected more than $5.6 billion in FCA and related recoveries during FY 2021, which is the second-largest total ever for FCA recoveries and the largest since 2014. That figure is inflated by Purdue Pharma’s $2.8 billion bankruptcy payment in connection with its opioid resolutions; but even without the Purdue payment, the government still recovered $2.8 billion from FCA defendants, in line with year-over-year trends for the last 5 years.
Meanwhile, on the legislative and policy front, the chief architect of the 1986 amendments, Senator Chuck Grassley (R-IA), advanced new legislation aimed at strengthening the FCA even further. DOJ also announced several of its own efforts to strengthen the FCA, including plans to unwind Trump Administration policies, leverage the FCA in cybersecurity enforcement, and police fraud on COVID-19 stimulus programs. On the judicial front, the federal appellate courts issued a number of significant decisions in the second half of 2021, including important decisions exploring the FCA’s materiality and scienter requirements, the public disclosure bar, and pleading fraud with particularity under Rule 9(b).
Below, we begin by summarizing recent enforcement activity, then provide an overview of notable legislative and policy developments at the federal and state levels, and finally analyze significant court decisions from the past six months.
As always, Gibson Dunn’s recent publications regarding the FCA may be found on our website, including in-depth discussions of the FCA’s framework and operation, industry-specific presentations, and practical guidance to help companies avoid or limit liability under the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
I. FCA ENFORCEMENT ACTIVITY
A. NEW FCA ACTIVITY
The government and qui tam relators filed 801 new cases in 2022.[1] That number is down from the unprecedented heights reached in 2020 (when there were a record 922 new FCA cases), but is consistent with the pace otherwise set over the past decade, reflecting the upward trend in FCA activity by qui tam relators and the government since the 2009 amendments to the statute.[2]
Last year, we noted that the government filed an abnormally high number of cases (250) on its own—i.e., without the involvement of qui tam relators. Cases of this sort remained historically high in 2021: although they dipped from 2020’s all-time high, DOJ initiated 203 cases in 2021, the second-highest total in the last 25 years. As discussed below, cases where the government is involved—either because the government brought the case, or later intervened—typically account for 90% of all FCA recoveries. If the government continues to bring more than 200 cases a year—up from an average of ~120 in the prior decade—then we will also expect to see increased DOJ recoveries. With the government’s stated commitments to proactive enforcement initiatives focusing on COVID stimulus fraud, cybersecurity, and matters stemming from health care data analysis, the number of government-driven new cases is likely to stay very high in 2022.
Number of FCA New Matters, Including Qui Tam Actions
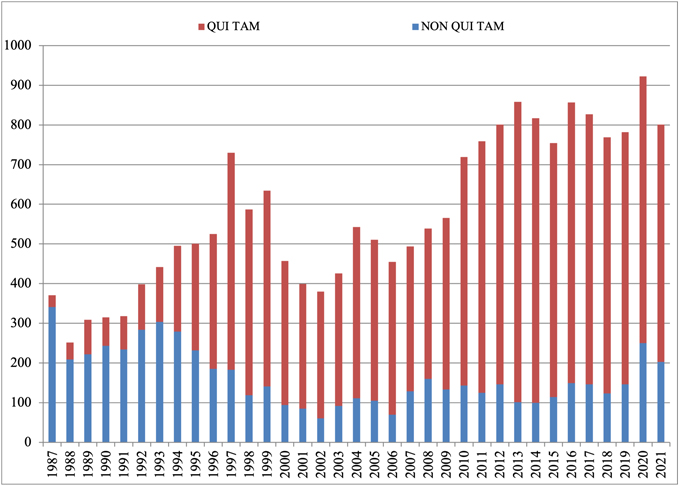
Source: DOJ “Fraud Statistics – Overview” (Feb. 1, 2022)
B. TOTAL RECOVERY AMOUNTS: 2021 RECOVERIES EXCEED $5.6 BILLION
The federal government recovered more than $5.6 billion during fiscal year 2021, which ended September 30, 2021. Of this amount, more than 90% was recovered in intervened cases, underscoring once again that companies face more significant exposure in cases in which the government initiated the case or intervened. Still, it is worth noting that relators’ recoveries from declined cases remained historically high since escalating significantly as a percentage of total recoveries in 2015; this data point reflects recent trends in relators’ willingness and ability to pursue cases into litigation after the government declines to intervene.
DOJ touted in its annual press release that the $5.6 billion haul represents the “second largest annual total in False Claims Act history, and the largest since 2014.”[3] But that only tells part of the story. The total includes approximately $3.2 billion in settlements stemming from the opioid crisis, including the $2.8 billion claim that Purdue agreed to allow in its bankruptcy “to resolve civil allegations that the company promoted its opioid drugs to health care providers it knew were prescribing opioids for uses that were unsafe, ineffective, and medically unnecessary, and that often led to abuse and diversion.”[4]
If the Purdue bankruptcy amount is removed, the government’s total recoveries this year are $2.8 billion. That amount is in line with trends during the last 5 years, while marking an increase from last year’s total of $2.2 billion.
Settlements or Judgments in Cases Where the Government Declined Intervention as a Percentage of Total FCA Recoveries
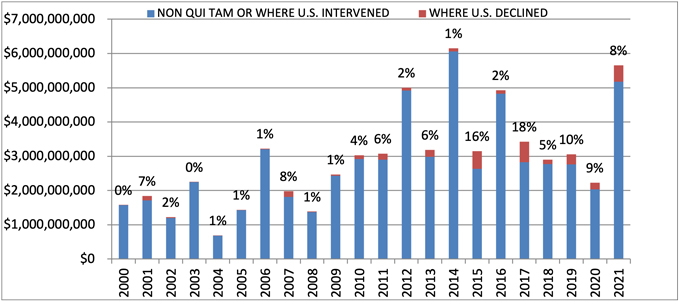
Source: DOJ “Fraud Statistics – Overview” (Feb. 1, 2022)
C. INDUSTRY BREAKDOWN
The relative breakdown of FCA recoveries across industries remained consistent with past years. Health care cases comprised 90% of total recoveries, Department of Defense procurement issues made up 2%, and the remaining 8% was split among other industries.[5] If you exclude the Purdue bankruptcy amount, then health care cases were 80% of recoveries, defense procurement cases were 4%, and other cases accounted for the remaining 16%.
Within the health care industry, DOJ announced that its primary areas of enforcement were opioid abuse, Medicare Advantage (Part C) fraud, illegal kickbacks, and provision of medically unnecessary services. While opioids, kickbacks, and provision of medically unnecessary services are familiar entries on the list of top theories for the government, the emergence of alleged Medicare Advantage fraud as one of DOJ’s top sources for FCA settlements is a relatively new development. Although close observers of the FCA have watched for several years as DOJ began to pursue these cases in earnest, those efforts are just beginning to result in significant recoveries for the government.
FCA Recoveries by Industry
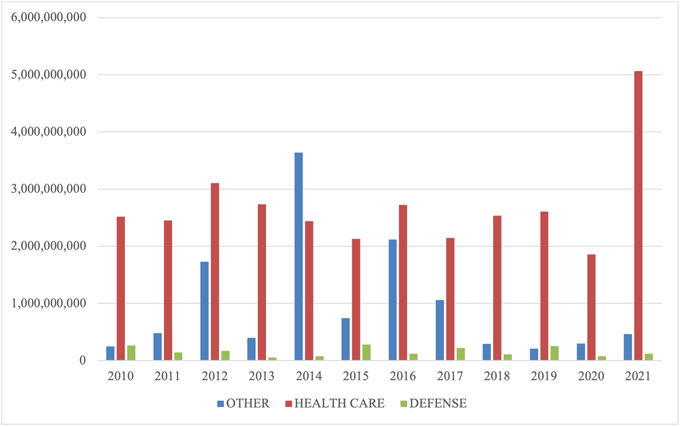
Source: DOJ “Fraud Statistics – Overview” (Feb. 1, 2022)
II. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE SECOND HALF OF 2021
We summarize below the most notable FCA settlements in the second half of calendar year 2021, with a focus on the industries and theories of liability involved. We covered settlements from the first half of the year in our 2021 Mid-Year Update.
A. HEALTH CARE AND LIFE SCIENCE INDUSTRIES
- On July 2, two rehabilitation therapy providers agreed to pay $8.4 million to resolve allegations that they violated the FCA by encouraging skilled nursing facilities in New York and New Jersey to bill Medicare for rehabilitation therapy services that were unnecessary, unreasonable, and performed by unskilled employees. The allegations stemmed from a qui tam suit filed by a former employee of the provider; the whistleblower’s share of the recovery was not reported with the settlement.[6]
- On July 2, an Ohio-based regional hospital system committed to pay $21.25 million to settle allegations that it paid certain physician groups substantially more than fair market value for patient referrals and then billed Medicare for these illegally referred patients, in violation of the Anti-Kickback Statute and the Stark Law. DOJ noted that the hospital system received credit for disclosing the allegedly improper compensation arrangements, which were implemented by the system’s prior leadership. The settlement also resolved claims brought under a qui tam suit filed by the hospital system’s former Director of Internal Audit; the whistleblower’s share of the recovery was not reported at the time of settlement.[7]
- On July 8, two related medical device manufacturers agreed to pay $38.75 million to resolve allegations that they violated the FCA by submitting, and causing others to submit, claims to Medicare for defective blood coagulation monitors (used by patients prescribed anticoagulant drugs to verify that they were taking a clinically appropriate and safe dosage of the medication). The government alleged that the manufacturers knew the monitors contained a material defect, which purportedly produced inaccurate and unreliable results, but nonetheless billed Medicare for use of the devices and did not take appropriate corrective action until the U.S. Food & Drug Administration (“FDA”) initiated a product recall.[8]
- On July 8, a medical device manufacturer committed to pay $27 million to settle allegations that it violated the FCA by knowingly selling defective miniature defibrillators to health care facilities that surgically implanted the devices into patients enrolled in federal health insurance programs. The government alleged that the manufacturer knew that the batteries in some of the defibrillators depleted prematurely and that two serious injuries and one death had been associated with premature battery depletion, but kept this information from the FDA when seeking approval for a change to the device to fix this issue. The settlement also resolved a qui tam suit filed by one of those patients; the whistleblower’s share of the recovery was not reported with the settlement.[9]
- On July 19, one of the country’s largest hospital systems, the system’s chief executive, and a referring physician entered into a $37.5 million joint settlement with DOJ and the California Department of Justice resolving federal and state fraud allegations. The settlement stemmed from two qui tam suits filed by former employees of the hospital system; the United States declined to intervene in those suits, but it participated in negotiating the settlement agreement. The agreement resolved allegations that the hospital system paid kickbacks to the referring physician in exchange for patient referrals; that the hospital system and the referring physician knowingly billed Medicare and Medi-Cal for another, suspended doctor’s services under the referring physician’s billing number; and that the hospital system and its chief executive knowingly overbilled Medi-Cal and two federal programs for implantable medical devices. As part of the settlement, the hospital system and its chief executive entered into a five-year Corporate Integrity Agreement requiring the system to maintain a compliance program and hire an Independent Review Organization to assess certain business transactions. One of the whistleblowers, a former hospital executive, will receive approximately $10 million of the recovery.[10]
- On July 23, a rehabilitation therapy provider in California agreed to pay $2 million to settle allegations that it caused false claims to be submitted to Medicare by pressuring therapists to artificially increase the number of patients who received Medicare’s most expensive level of care for skilled nursing, “Ultra High,” even when that level of services was not necessary. The settlement resolved a qui tam suit filed by a former Director of Rehabilitation at the company, who will receive $360,000 of the settlement proceeds.[11]
- On July 21, an ambulatory electroencephalography (“EEG”) testing company and a private investment firm that owned a minority share in the company, both based in Texas, agreed to pay $15.3 million to settle kickback and false billing allegations. The United States alleged that the testing company improperly: induced physicians to refer patients to the company in exchange for free EEG test-interpretation reports; inflated invoices for certain EEG testing by using inaccurate billing codes; and billed for a specialized digital analysis that it never performed. The United States also alleged that the private investment firm learned about these schemes when performing pre-investment due diligence but allowed the schemes to continue. The settlement agreement consisted of payments to both the federal government and state Medicaid programs and also resolved claims brought under six different qui tam Two of the relators received approximately $3 million of the settlement funds; the respective shares of the other relators were not included in the settlement announcement.[12]
- On July 20, a Billings, Montana rheumatologist committed to pay $2 million to resolve allegations that he violated the FCA by billing Medicare for improper medical treatments. The United States asserted that the rheumatologist knowingly billed Medicare for MRI scans, patient visits, and biologic infusions on behalf of patients who did not have rheumatoid arthritis.[13]
- On August 2, a recently closed mail-order diabetic testing supplier and its parent company agreed to pay $160 million to resolve allegations that they violated the FCA through a kickback scheme and other fraudulent billing practices. The government alleged that the testing supplier, with its parent company’s approval, paid kickbacks to Medicare beneficiaries in the form of free glucometers and waived co-payments; billed Medicare for glucometers for which patients were not eligible; and billed Medicare on behalf of deceased patients, which led Medicare to revoke the company’s supplier number in 2016. In 2019, the testing company’s two founders each paid $500,000 to resolve allegations that they were personally involved in the kickback scheme. The civil settlement also resolves a qui tam suit filed by a former employee who worked at the testing company’s call center; he will receive approximately $28.5 million as his share of the recovery.[14]
- On August 5, a hospital system in Michigan agreed to pay $2.8 million to resolve allegations that it violated the FCA by submitting bills to federal health care programs for medically unnecessary procedures performed by a specific physician. In the settlement announcement, DOJ noted that the hospital system made a submission under the Provider Self-Disclosure Protocol of the U.S. Department of Health & Human Services, Office of Inspector General (“HHS-OIG”) and implemented several remedial measures to address the physician’s conduct, such as hiring another physician to conduct a peer review, placing the physician in a performance improvement plan, and ultimately ending its relationship with the physician. The settlement also resolved a qui tam suit filed by three individuals, who received a combined payment of $532,000 as their share of the recovery.[15]
- On August 6, a county medical center in California agreed to pay $11.4 million to settle allegations that it submitted, or caused the submission of, false claims to Medicare by admitting Medicare beneficiaries for inpatient services that were not medically necessary, such as when no alternative placements for a beneficiary could be found. As a part of the settlement, the medical center entered into a Corporate Integrity Agreement requiring the center to hire an Independent Review Organization to annually review the medical center’s inpatient admissions and billings of federal health insurance programs. The settlement also resolved a qui tam suit filed by a former employee of the medical center; that individual’s share of the recovery was not reported with the settlement announcement.[16]
- On August 25, a California-based provider of home respiratory services and durable medical equipment agreed to pay $3.3 million total to the United States, California, and Nevada to settle allegations that it violated the FCA by billing public health care programs for home ventilators that were not medically necessary or that patients were no longer using. The settlement also resolved a qui tam suit filed by a respiratory therapist who worked for the company, who will receive approximately $612,000 as his share of the settlement amount.[17]
- On August 26, a mental health and addiction treatment services provider agreed to the entry of judgments totaling over $15 million to resolve allegations that it violated the FCA and the Controlled Substances Act. Approximately $13.7 million, plus interest, of the judgment went to resolving allegations that the provider billed Medicare and Medicaid for mental health services performed by unqualified practitioners and billed Medicaid for mental health services using incorrect procedure codes that inflated the price of the care provided. The settlement partially resolved a related whistleblower suit filed by former employees, although the relators in that suit continued to pursue additional FCA claims against the company and its former chief executive. Additionally, the company agreed to the entry of a judgment for approximately $1.6 million, plus interest, to resolve allegations that it negligently failed to document its use of controlled substances and its transfer of controlled substances between locations. DOJ continued to pursue claims against several executives, including the former chief executive, alleging violations of the Controlled Substances Act.[18]
- On August 27, a hospital in Texas agreed to pay $3.3 million to settle a qui tam suit brought by its former Director of Compliance resolving allegations that the hospital improperly utilized billing modifiers to inflate the cost of care it billed to federal health care programs. The relator received approximately $912,000 of the recovery. The settlement announcement noted that, although the government did not intervene in the case, it investigated the relator’s allegations and collaborated with the relator.[19]
- On August 30, a California-based company who contracted to provide health care services to certain Medicare Advantage patients committed to pay $90 million to resolve allegations that it knowingly submitted incorrect diagnosis codes for those patients who inflated federal payments made to Medicare Advantage plans and, in turn, the provider. The settlement also resolved a qui tam suit filed by a former employee of the provider; the United States intervened in the claims against some, but not all, of the defendants named in the suit. Also, pursuant to the settlement, the provider entered into a five-year Corporate Integrity Agreement requiring it to implement a risk assessment program into its overarching compliance program and hire an Independent Review Organization to review a sample of its Medicare Advantage patients’ medical records and diagnosis codes each year.[20]
- On September 3, the U.S. District Court for the District of South Carolina entered default judgments totaling $140 million against various pain management clinics, drug testing laboratories, and a substance abuse counseling center. The government alleged the defendant entities violated the FCA by providing illegal financial incentives to providers to induce drug test referrals and billed the federal government for unnecessary drug tests. The allegations arose from qui tam complaints by five former employees of the defendant pain management clinics.[21]
- On September 8, a collection of home health care companies agreed to pay $17 million to settle allegations that they violated the FCA by paying a kickback to a retirement home operator when they purchased two of its home health agencies. The government alleged the companies bought the home health agencies to induce the seller to refer Medicare beneficiaries residing in its retirement homes throughout the United States, and then submitted false claims to Medicare for the resulting services. The allegations stem from a qui tam complaint, and the whistleblower’s share of the recovery was not disclosed at the time of the settlement announcement.[22]
- On September 15, a cardiologist agreed to pay $6.75 million to settle allegations that he violated the FCA by billing federal health care programs for medically unnecessary ablations and vein stent procedures performed on patients. The government alleged that the cardiologist made false statements in patient medical records to justify the procedures, and that many of the ablations were performed by ultrasound technicians outside the scope of their practice. The cardiologist and his consulting company entered into a multi-year integrity agreement including requirements for training, reporting, and independent quarterly claims reviews.[23]
- On October 1, three pharmaceutical manufacturers agreed to pay $447.2 million to resolve allegations that they violated the FCA by conspiring to fix prices for various generic drugs, resulting in higher drug prices for federal health care programs. DOJ alleged a novel theory of remuneration, contending that the manufacturers conveyed value in the form of agreements on pricing. The settlement is in addition to $424.7 million previously paid by the companies to resolve related criminal charges. The government alleged that between 2013 and 2015, the companies entered agreements on pricing, supply, and allocation of customers with other pharmaceutical manufacturers for drugs manufactured by the companies, purportedly in violation of the Anti-Kickback Statute. Each company also entered into a five-year Corporate Integrity Agreement, which includes monitoring, price transparency, and other compliance provisions.[24]
- On October 8, two providers of home-based health care services agreed to pay $8.5 million to settle allegations that they violated the FCA by submitting claims to Medicare for laboratory and diagnostic testing performed between 2010 and 2015 that was not medically necessary. The allegations arose from five qui tam lawsuits; the first-to-file whistleblower will receive $1.53 million as a result of the settlement.[25]
- On October 22, two physicians agreed to pay $3.9 million to settle allegations that they violated the FCA by ordering unnecessary drug tests for patients at their pain management clinic and lab. The allegations stem from a lawsuit filed by two qui tam The relators will receive 17% of the United States’ portion of the settlement, totaling approximately $618,000.[26]
- On October 25, a group of pharmacies agreed to pay $4.6 million to resolve allegations that the pharmacies violated the FCA in various ways, including by charging the government higher prices than those charged to other patients and paying kickbacks to a third-party marketer who assisted in arranging for medically unnecessary prescriptions of pain and scar creams. The allegations arose from a qui tam lawsuit filed by a former pharmacist. The whistleblower will receive approximately $800,000 from the settlement.[27]
- On October 28, a physical therapy company agreed to pay $4 million to settle allegations that it violated the FCA by billing the government for outpatient physical therapy that was not provided. The allegations stem from a qui tam lawsuit, and the relator’s share was not disclosed at the time of the settlement announcement.[28]
- On November 8, a medical device company agreed to pay $16 million to resolve allegations that it violated the FCA by causing the submission of false claims to Medicare. The company made royalty payments to an orthopedic surgeon related to the surgeon’s contributions towards the company’s products. However, according to the government, these payments constituted illegal kickbacks because they were allegedly intended to encourage the surgeon to use and recommend the company’s products to patients. The underlying allegations relate to a qui tam suit, and the whistleblower will receive approximately $2.5 million of the settlement payment.[29]
- On November 9, several anesthesia providers and outpatient surgery centers agreed to pay more than $28 million to resolve allegations that they violated the FCA by entering into arrangements involving purported kickbacks. The government alleged that the anesthesia providers sought to enter exclusive contracts with the outpatient surgery centers by offering kickbacks in the form of payments for drugs, supplies, and equipment and labor, as well as through the provision of free staffing services. According to the government, the alleged kickback scheme caused the submission of false claims. Relators who brought the underlying qui tam suit will receive over $4.7 million of the settlement payment.[30]
- On November 9, a pharmaceutical manufacturer agreed to pay $12.7 million to resolve allegations that it violated the FCA by causing the submission of false claims for an injectable drug. The company allegedly directed doctors to preferred pharmacies despite being aware that certain of those preferred pharmacies submitted false prior authorization requests that misrepresented to insurers that the requests were submitted by physicians instead of by the pharmacies themselves, and/or included false or misleading information about the underlying patients’ medical histories. The allegations relate to a qui tam suit, and the whistleblower will receive approximately $2.5 million of the settlement payment.[31]
- On November 22, a chiropractor agreed to a $9 million civil consent judgment to resolve allegations that he violated the FCA by conspiring to pay illegal kickbacks and bill for unnecessary medical services. The chiropractor purportedly caused the submission of false claims to federal health care programs through kickbacks paid to physicians for urine drug testing referrals, as well as through medically unnecessary prescriptions for pain creams. The chiropractor pleaded guilty to criminal charges arising from the same conduct and faces a potential sentence of up to five years in prison and a fine of $250,000.[32]
- On November 22, a home health agency agreed to pay $4.2 million to resolve allegations that it violated the FCA by submitting claims for services that were not covered by Medicare or Medicaid, and by failing to timely refund associated overpayments. The non-covered services underlying the allegedly false claims included, among other things, services that lacked the required face-to-face certifications or plans, and services that did not document the beneficiary’s need for home care. The underlying allegations relate to a qui tam suit, and the relator will receive over $700,000 from the settlement.[33]
- On November 23, a hospice and palliative care provider operating in Ohio and Tennessee agreed to pay $5.5 million to settle allegations that it violated the FCA by submitting claims to Medicare for non-covered hospice services. The provider allegedly submitted false claims to Medicare between January 2012 and December 2014 for hospice services and care provided to patients who were not terminally ill for at least a portion of the relevant time period during which they received care. The allegations arise from a qui tam lawsuit, and the whistleblower will receive approximately $1 million of the settlement payment.[34]
- On December 2, a hospital agreed to pay $18.2 million to resolve allegations that it violated the FCA by submitting claims to Medicare, Medicaid, and TRICARE for services referred to the hospital as a result of alleged kickbacks. The hospital allegedly induced certain physicians to refer patients to the hospital by giving those physicians shares repurchased from older physician-owners. According to the government, the hospital then violated the FCA by submitting claims for services referred or ordered by the physicians who received the newly repurchased shares. The underlying allegations stem from a qui tam lawsuit, and the associated whistleblower will receive approximately $3 million of the settlement payment.[35]
- On December 7, a pathology practice agreed to pay $2.4 million to resolve allegations that it violated the FCA by making false representations in connection with submissions to the government. The practice allegedly submitted certain claims to the government without written substantiation, and as a result billed Medicare for a type of testing analysis that was not actually required. Contemporaneous with the settlement, the pathology practice entered into a three-year Corporate Integrity Agreement requiring training, auditing, and monitoring designed to address the alleged misconduct along with other evolving compliance risks. The underlying allegations arose from a qui tam lawsuit, and the whistleblower for that suit will receive approximately $450,000 from the settlement.[36]
- On December 8, a physician and a medical device manufacturer agreed to pay a collective $4.2 million to resolve allegations that they violated the FCA by entering into kickback arrangements. The government alleged that, between 2014 and 2018, the company provided kickbacks—in the form of cash payments, commissions, and all-expense paid trips—to the physician to induce him to direct physicians at his institute to utilize the company’s medical devices and to increase the number of certain surgeries performed in order to then increase orders of the company’s supplies. The physician also allegedly directed physicians to order toxicology and genetic tests from a medical testing laboratory from which he accepted additional kickbacks. The civil settlement resolved claims brought under a qui tam suit, and the whistleblower will receive approximately $600,000 from the settlement.[37]
B. GOVERNMENT CONTRACTING AND PROCUREMENT
- On July 6, an aviation company based in Illinois and Florida agreed to pay $11 million to resolve allegations that it violated the FCA in connection with two U.S. Transportation Command contracts for aircraft maintenance services supporting U.S. Department of Defense (“DOD”) operations in Afghanistan and Africa. According to the government, the company knowingly failed to maintain several helicopters utilized by the Department of Defense to transport cargo and personnel in accordance with contract requirements, such that the helicopters were not airworthy and should not have been certified as “fully mission capable.” The settlement included a resolution of claims brought in a qui tam suit filed by a former employee, who received approximately $2.2 million of the settlement proceeds. The company also agreed to pay a separate $429,000 fine to the Federal Aviation Administration for purported deficiencies in its helicopter maintenance program.[38]
- On September 27, an oil and natural gas exploration and production company agreed to pay $6.15 million to settle allegations that it violated the FCA by underpaying royalties for natural gas from federal lands. The government alleged that the company improperly deducted processing costs from the royalties due to the United States under a lease that permitted the company to extract natural gas from federal lands as long as the gas was placed in marketable condition at no cost to the United States.[39]
- On October 6, a military supplier agreed to pay over $4.5 million to settle allegations that it violated the FCA by failing to comply with requirements for certain products supplied to the military. The government alleged that the supplier had provided high-performance butterfly valves to military ship builders from May 2011 through September 2017 and failed to disclose that there had been unapproved modifications made to the valves. The allegations stem from a qui tam lawsuit brought by a former employee, and the whistleblower will receive approximately $850,000 of the recovery.[40]
- On October 15, a contracting company entered into a consent judgment, agreeing to pay $4.8 million to settle allegations that it violated the FCA by submitting false certifications of eligibility to obtain federal contracts intended to benefit service-disabled veterans. The complaint alleged that the company was not owned by a veteran but instead recruited a service-disabled veteran to nominally run a pass-through entity that enabled the company to obtain federal contracts for which it otherwise would not have been eligible.[41]
- On December 21, an information technology contractor agreed to pay over $1.3 million to resolve allegations that it violated the FCA when seeking payment for information technology and cybersecurity services. The government alleged that the company caused the submission of false claims by billing the government for labor hours in excess of time worked, labor rates that exceeded the rates actually paid to employees, labor costs exceeding the company’s actual recorded costs, and overly high indirect rates.[42]
- On December 22, an aircraft manufacturer agreed to pay $1.9 million to resolve allegations related to a jet fuel spill, including allegations that it violated the FCA during the government’s investigation of the incident. According to the government, employees of the manufacturer made material false statements to avoid contractual liability for cleanup related to the spill and, by doing so, violated the reverse false claims provision of the FCA.[43]
C. OTHER
- On July 12, the Florida Department of Children and Families (“FDCF”) agreed to pay $17.5 million to settle allegations that its management of federal Supplemental Nutrition Assistance Program (“SNAP”) funds violated the FCA, one of several settlement agreements the United States has secured recently with state agencies and a private consulting firm regarding alleged manipulation of SNAP data. The U.S. Department of Agriculture (“USDA”) requires states to determine individuals’ eligibility for SNAP benefits and maintain quality control processes to confirm eligibility decisions. Additionally, states must accurately report their error rates in awarding benefits to USDA, and USDA pays performance bonuses to states that report low error rates and demonstrate decreasing error rates. The government alleged that FDCF improperly injected bias into its SNAP quality control program that caused the submission of false information to USDA regarding FDCF’s error rate. This, in turn, allegedly led USDA to pay FDCF performance bonuses that it did not earn. As part of the settlement, FDCF also agreed to forego $14.7 million in pending bonus payments that it had not yet received from USDA.[44]
- On July 28, two clothing companies and their former chief executive and owner agreed to pay $6 million to resolve allegations of underreporting the value of imports on invoices submitted to U.S. Customs & Border Protection in order to pay less in customs duties. In a related criminal prosecution from 2020, the former chief executive and owner also pled guilty to a subset of this conduct, was sentenced to six months in prison, and paid a separate forfeiture amount of $1.7 million. The allegations leading to his conviction and the subsequent civil settlement agreement stemmed from a qui tam complaint, but the whistleblower share of the recovery was not announced with the settlement.[45]
- On August 5, the Tennessee Department of Human Services (“TDHS”) committed to pay $6.8 million to resolve allegations that it submitted false quality control data to USDA regarding TDHS’s award of SNAP benefits to low-income individuals. The United States alleged that TDHS, in implementing the recommendations of an outside consulting firm, inserted bias into its quality control program that led to the submission of false quality control data to USDA. According to the government, TDHS then received performance bonuses from USDA on the basis of this false data.[46]
III. LEGISLATIVE AND POLICY DEVELOPMENTS
A. FEDERAL POLICY DEVELOPMENTS
In its first year, the Biden Administration has not ushered in a major shift in overarching FCA policy. But the Administration has emphasized that it remains focused on FCA enforcement, and significant changes have nonetheless begun to take shape.
1. Biden Administration Continues Move Away from “Brand” Memo
In July 2021, DOJ promulgated an interim final rule that opens the door for DOJ attorneys to leverage agency guidance in enforcement actions. This step culminates a shift that we flagged in our 2021 Mid-Year Update, in which we discussed Executive Order 13992, issued on the day of President Biden’s inauguration. That Order foreshadowed a possible shift away from DOJ’s so-called Brand Memo.
By way of background, the internal DOJ guidance communicated by the January 2018 Brand Memo stated that agency “[g]uidance documents” without notice-and-comment rulemaking “cannot create binding requirements that do not already exist by statute or regulation.”[47] In December 2018, DOJ codified the Brand Memo at Section 1-20.100 of the Justice Manual, which explained that with limited exceptions, DOJ “should not treat a party’s noncompliance with a guidance document as itself a violation of applicable statutes or regulations.”[48] Executive Order 13992 did not refer expressly to DOJ’s civil enforcement of the FCA, but it did explicitly revoke President Trump’s Executive Order 13891, which expressed a principle similar to that codified in Section 1-20.100 of the Justice Manual.
Effective July 1, 2021, DOJ issued the interim final rule implementing Executive Order 13992 and rescinding DOJ regulations that proscribed use of guidance documents in DOJ enforcement actions.[49] On the same date, Attorney General Merrick Garland issued a memo to all DOJ component heads explaining that, while guidance alone cannot form the basis for an enforcement action, it “may be entitled to deference or otherwise carry persuasive weight with respect to the meaning of the applicable legal requirements” in a particular case.[50] The memo stated that “[t]o the extent guidance documents are relevant to claims or defenses in litigation, Department attorneys are free to cite or rely on such documents as appropriate.”[51] These developments surely will open back up the reliance that many DOJ attorneys placed on sub-regulatory guidance as evidence both that a claim is materially false and that defendants recklessly disregarded statutory and regulatory requirements. On the other hand, while the Brand Memo’s safeguards against the use of guidance as the basis for asserting falsity under the FCA have eroded, certain federal courts—and Supreme Court justices—have signaled significant skepticism about use of sub-regulatory guidance to impose substantive legal standards. E.g., Whitman v. United States, 574 U.S. 1003 (2014) (statement of Justice Scalia and Justice Thomas on denial of certiorari, warning that courts should not defer to “executive interpretations of a variety of laws that have both criminal and administrative applications”).
This is a development we will continue to closely watch.
2. DOJ Announces Initiative to Use FCA in Cybersecurity Cases
On October 6, 2021, Deputy Attorney General Lisa Monaco announced a new Civil Cyber Fraud Initiative that will leverage the FCA to “hold accountable entities or individuals that put U.S. information or systems at risk by knowingly providing deficient cybersecurity products or services, knowingly misrepresenting their cybersecurity practices or protocols, or knowingly violating obligations to monitor and report cybersecurity incidents and breaches.”[52] The new effort will “combine [DOJ’s] expertise in civil fraud enforcement, government procurement and cybersecurity to combat new and emerging cyber threats to the security of sensitive information and critical systems.”[53]
On October 13, 2021, Acting Assistant Attorney General Brian Boynton delivered remarks on this new initiative at the Cybersecurity and Infrastructure Security Agency Fourth Annual National Cybersecurity Summit.[54] AAG Boynton noted that the Civil Cyber Fraud Initiative “will use the [FCA] to identify, pursue and deter cyber vulnerabilities and incidents that arise with government contracts and grants and that put sensitive information and critical government systems at risk.” He indicated that “three common cybersecurity failures” are “prime candidates” for potential FCA enforcement by DOJ: (1) “knowing failures to comply with cybersecurity standards,” such as those negotiated with government contractors; (2) “knowing misrepresentation of security controls and practices”; and (3) “knowing failure to timely report suspected breaches.”
3. DOJ Continues FCA Enforcement to Protect COVID-19 Spending
Spending packages tied to COVID-19 also continue to be a focus for federal FCA enforcement. Under the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”)[55] and subsequent stimulus programs, including the American Rescue Plan in March 2021,[56] the government provided trillions of dollars in government funds to mitigate the effects of COVID-19. Anytime the government spends money, particularly massive amounts, there is a possibility the funds are misspent. This, in turn, brings the FCA into play as a potential remedy; unsurprisingly, the government has repeatedly indicated its intent to rely heavily on the FCA to police any fraud associated with pandemic relief funds.
The Paycheck Protection Program (“PPP”), one of several programs created by the CARES Act, aims to allow businesses to receive low-interest private loans to pay for their payroll and certain other costs. In January 2021, DOJ settled its first civil case involving the Paycheck Protection Program with SlideBelts, Inc. and its President and CEO.[57] Since then, DOJ has entered into at least two other settlements related to the Program.
- Pursuant to the settlement agreement reached in United States ex rel. Hablitzel v. All in Jets, LLC & Seth A. Bernstein, the owner of a jet charter company agreed to pay almost $290,000 to settle allegations that he diverted nearly $100,000 from a $1.2 million PPP loan for personal, non-company related expenses within a day of receiving the loan. The relator (a former employee) who initially filed the action received close to $60,000.[58]
- In United States ex rel. Quesenberry v. Sextant Marine Consulting LLC et al., a duct cleaning company agreed to pay $30,000 in damages and civil penalties for conduct associated with two PPP loans, in addition to repaying the duplicative PPP funds in full.[59] The relator received $4,500 of this amount. According to DOJ, additional claims against other entities remain under seal in Quesenberry.
These sorts of relatively small dollar-value settlements likely do not represent the extent of DOJ’s focus on FCA enforcement related to PPP funds. FCA investigations—and subsequent litigation—often take years to play out. And all signs indicate that the biggest and most important FCA cases to come out of the COVID-19 pandemic are still to come.
The CARES Act also contained a provision establishing a Special Inspector General for Pandemic Recovery (“SIGPR”),[60] who “has the duty to conduct, supervise, and coordinate audits and investigations of the making, purchase, management, and sale of loans, loan guarantees, and other investments by the Secretary of the Treasury under any program established by the Secretary under Division A of the CARES Act.”[61] Since September 2020, the SIGPR has added thirty-three staff members, and it has sought “a place in the annual federal budget” beyond the original $25 million first allocated for five years of the office’s service.[62] The SIGPR has thus far focused on cases where CARES Act participants allegedly sought funding from multiple programs to be used for the same purpose.[63] Although the SIGPR has not focused on FCA claims, the fact that it has referred a significant number of fraud-related claims to other agencies for investigation leaves the door open to new FCA claims arising from this office.
4. DOJ Updates FCA Penalty Amount to Adjust for Inflation
DOJ is required by law to adjust penalties to keep pace with inflation. In December, DOJ issued a final rule to update penalties for 2021. See 86 Fed. Reg. 70740 (Dec. 13, 2021). Under the final rule, FCA penalties now range from a minimum of $11,803 to a maximum of $23,607 for penalties assessed after December 13, 2021, compared to the prior range of $11,665–$23,331. These increased amounts apply to any violation of the FCA that occurred after November 2, 2015, when the law requiring inflation adjustments took effect.
B. FEDERAL LEGISLATIVE DEVELOPMENTS
Congressional attention on FCA enforcement reignited in the latter half of 2021 with the introduction of proposed legislative amendments by Senator Chuck Grassley (R-IA). Senator Grassley, the architect of the 1986 amendments, has long touted himself as a leading advocate of the FCA. This past year, he set his sights on cementing his legacy as the FCA’s principal champion.
As discussed in our Mid-Year Update, in February 2021, Senator Grassley sent a letter to then-Attorney General Nominee Merrick Garland, criticizing DOJ’s actions in dismissing relator claims, decrying the effects of the Supreme Court’s decision in Escobar, and requesting assistance in crafting new legislation to address his concerns.[64]
In July 2021, Senator Grassley—joined by a bipartisan group of four co-sponsors—made good on his promise and introduced the False Claims Amendments Act of 2021 (the “Amendments”), which included four main provisions.[65] First, the Amendments would implement a burden-shifting scheme under which the plaintiff (government or relator) may establish materiality by a simple “preponderance of the evidence,” at which point the defendant “may” rebut evidence of materiality by “clear and convincing” proof. Second, the Amendments would require that, when DOJ seeks to dismiss declined qui tam cases, the relator must first have an opportunity to show that the reasons for dismissal are fraudulent, arbitrary and capricious, or contrary to law. Third, the Amendments would allow DOJ to shift the cost of discovery defendant served on the government to the defendant if the defendant’s discovery requests are irrelevant, disproportional, or unduly burdensome. Finally, the Amendments would expressly apply the FCA’s existing anti-retaliation provisions to post-employment retaliation. The Amendments would be applicable to all pending and future litigation “to ensure that [the FCA] covers the trillions of dollars spent on COVID relief.”[66]
In October 2021, Senator Grassley amended his proposal in several respects. First, he eliminated the burden-shifting approach to the materiality standard. This portion of the bill now reads: “In determining materiality, the decision of the Government to forego a refund or pay a claim despite actual knowledge of fraud or falsity shall not be considered dispositive if other reasons exist for the decision of the Government with respect to such refund or payment.”[67] With this revision, Senator Grassley again is targeting the Supreme Court’s decision in Escobar.
If adopted, this provision could have broader implications than the original burden‑shifting framework. The original framework, by its plain language, focused only on evidentiary burdens, whereas the revised framework speaks in more general terms of “determin[ations]” of materiality.[68] Such determinations could occur at the motion-to-dismiss stage, as the Escobar Court clearly contemplated, even before litigation stages involving evidentiary determinations. A basic principle of civil pleading is that an “obvious alternative explanation” for the conduct a plaintiff alleges is enough to make the plaintiff’s allegations legally implausible and subject to dismissal. See Bell Atl. Corp. v. Twombly, 550 U.S. 544, 567–68 (2007). The revised provision of the Amendments threatens to upend this principle by making payment of claims notwithstanding their falsity “not . . . dispositive” of materiality as a matter of law, where otherwise such facts could form the basis for an argument that an “obvious alternative explanation” for the government’s payments was that it spotted the alleged fraud and decided it was immaterial. Senator Grassley’s amendments also removed the original bill’s cost-shifting provisions for discovery expenses.[69]
This revised bill was reported out of Committee in November 2021,[70] but it remains unclear if the bill will receive a vote on the Senate floor. If enacted, however, the Amendments would surely give DOJ and relators new arguments in a variety of different FCA scenarios, and could have a meaningful impact on the cost of defending against FCA cases, particularly including meritless cases brought by a would-be whistleblower.
Gibson Dunn will continue to monitor this and other legislative developments.
C. STATE LEGISLATIVE DEVELOPMENTS
The federal government provides incentives for states to enact false claims statutes in keeping with the federal FCA. In particular, HHS-OIG grants “a 10-percentage-point increase” in a state’s share of any recoveries under the relevant laws to any state that obtains HHS-OIG approval for its false claims statute.[71] Such approval requires that the statute in question, among other requirements, “contain provisions that are at least as effective in rewarding and facilitating qui tam actions for false or fraudulent claims as those described in the [federal] FCA.”[72] Approval is also contingent on the statute containing a sixty-day sealing provision and “a civil penalty that is not less than the amount of the civil penalty authorized under the [federal] FCA.”[73] The total number of states with approved statutes remains at twenty-two, with Montana (which was previously on the “approved” list) having obtained approval on October 4, 2021 for the amendments to its false claims act that we covered in our 2021 Mid-Year Update.[74] The list of seven states with false claims statutes listed by HHS-OIG as “not approved” likewise remains the same: Florida, Louisiana, Michigan, New Hampshire, New Jersey, New Mexico, and Wisconsin.[75]
The “not approved” list may soon shrink, however. On October 20, 2021, the Wisconsin legislature introduced a bill that would “restore[] a private individual’s authority to bring a qui tam claim against a person who makes a false claim for medical assistance, which was eliminated in 2015 Wisconsin Act 55.”[76] The bill also provides for qui tam awards of up to thirty percent of recoveries, which is consistent with the federal FCA, and for “additional changes not included in the prior law to conform state law to the federal [FCA], including expanding provisions to facilitate qui tam actions and modifying the bases for liability to parallel the liability provisions under the federal False Claims Act.”[77] While Wisconsin’s 2015 repeal of false claims liability for Medicaid claims was not expressly one of the grounds HHS-OIG relied on in refusing to grant federal incentives, the government did in 2016 cite the state statute’s lack of a provision pegging civil penalties to inflation as grounds for the government’s continued “not approved” designation.[78] The 2021 bill ostensibly seeks to address this issue (among others cited by HHS-OIG) by incorporating the federal FCA penalty amounts, which are pegged to inflation.[79]
IV. CASE LAW DEVELOPMENTS
The second half of 2021 saw a number of notable federal appellate court decisions, which we have summarized below.
A. Seventh and Second Circuits Evaluate How Government’s Continued Payment Bears on Materiality Under Escobar
During the latter half of 2021, courts continued to refine the FCA’s materiality requirement, applying the standard articulated by the Supreme Court in Universal Health Services Inc. v. United States ex rel. Escobar, 579 U.S. 176 (2016). In Escobar, the Supreme Court noted that to survive a motion to dismiss plaintiffs must “plead[] facts to support allegations of materiality” when bringing claims under the FCA. Id. at 195 n.6. In the five years since Escobar, materiality has become a key consideration at the motion-to-dismiss stage, even when it requires courts to make factually specific, case-by-case determinations.
In United States v. Molina Healthcare of Illinois, Inc., 17 F.4th 732 (7th Cir. 2021), the Seventh Circuit reversed a district court’s order dismissing a complaint involving alleged false certifications by Molina Healthcare of Illinois, Inc. relating to Medicaid reimbursement. Molina Healthcare and the State of Illinois had agreed to a capitated system of Medicaid payments—payments based on the number of patients a health care provider projects it will serve based on patients’ risk levels. Id. at 736. As part of the agreement, Molina agreed to provide skilled nursing facility services to the most expensive (riskiest) tier of patients. Id. A relator filed an FCA complaint alleging that Molina falsely certified that it was providing such services to patients. According to the relator, Molina stopped providing these services a year into the agreement, yet continued to collect the Medicaid payments owed under the capitated payment system for the most expensive tier of patients. Id. at 737.
The Seventh Circuit allowed the relator’s suit to proceed, concluding that Molina Healthcare’s certification regarding its skilled nursing facility services was material to the State’s decision to continue making Medicaid payments to Molina Healthcare. Id. at 744. The court acknowledged that the State continued paying even after it knew that Molina Healthcare did not provide the relevant services. Id. at 743–44. But it concluded this did not dispose of the materiality inquiry. Rather, the court focused on the fact that those patients who qualified for skilled nursing facility services were in a much more expensive tier in the capitation system than other patients. Id. at 743. The difference in cost between patients in the most expensive tier and those in lower tiers suggested that Molina Healthcare’s certification was material, notwithstanding the inference that the government knew of the alleged misconduct and disregarded it in its payment decision.
The court also held that the relator adequately alleged Molina Healthcare knew its certifications were material. Id. at 745. The district court had also premised its dismissal on the relator’s failure to plead specific allegations about Molina Healthcare’s knowledge. The Seventh Circuit disagreed. It concluded that because Molina Healthcare was “a highly sophisticated member of the medical-services industry,” relator plausibly alleged that the company may have known the importance of its false certifications in Medicaid payment decisions. Id. at 744-5.
In United States ex rel. Foreman v. AECOM, 19 F.4th 85 (2d Cir. 2021), the Second Circuit applied Escobar’s materiality factors and affirmed, in part, a district court’s decision to dismiss a relator’s FCA complaint. The case involved allegations that AECOM, a company that provided maintenance and management services to the U.S. Army in Afghanistan, violated the FCA by failing to live up to contractual obligations. Id. at 95. Specifically, the relator alleged that AECOM overstated its man-hour utilization rate, overbilled the government for labor not actually performed, and failed to properly track government property. Id. AECOM moved to dismiss, arguing that the alleged misrepresentations to the government were not material to any payments, pointing to government reports suggesting that the government knew about the issues and continued to pay in any event. The district court agreed and dismissed the complaint.
The Second Circuit affirmed in part and reversed in part. In doing so, the Second Circuit focused on three factors identified in Escobar to determine whether a false certification of compliance with a contract was material to payment by the government: (1) whether the government “expressly identified a provision as a condition of payment,” id. at 110, (2) “the government’s response to noncompliance” with the contract, id. at 111, and (3) whether the “alleged noncompliance was substantial,” id. at 116. In granting AECOM’s motion to dismiss, the district court had focused on the second factor, relying on government reports indicating that the government had been aware of the alleged false claims and still chose to pay AECOM for its work under the contract.
Although the Second Circuit observed that the government’s continued payment—i.e., the “response to noncompliance”—bore heavily on the question of materiality, the court took a restrictive view of what documents it could consider at the motion-to-dismiss stage to demonstrate the government’s “response to noncompliance.” In drafting the complaint, the relator had expressly referenced and relied on government reports about man-hour-utilization rate and property tracking. Id. at 113–14. Because the relator’s complaint included those documents, the Second Circuit determined that the trial court could consider these documents. And those reports indicated that the government knew about the alleged fraud and “not only continued to extend and pay claims under the [contract], but also never demanded repayment, disallowed any charged costs, or penalized AECOM.” Id. at 115. By contrast, the court concluded that separate government reports referencing overbilling—which the district court also relied on—were not integral to or referenced in the complaint and, thus, should not have been considered at the pleading stage. Id. at 116. Without the presence of those reports for consideration on the motion to dismiss stage showing the government’s response to AECOM’s alleged overbilling practices, the court allowed the relator to proceed on those claims. Id. at 117–18.
B. Seventh and Eighth Circuits Issue Important Decisions on the FCA’s Scienter and Falsity Requirements
In United States ex rel. Schutte v. SuperValu, Inc., 9 F.4th 455 (7th Cir. 2021), the Seventh Circuit became the latest circuit court to apply the scienter standard announced by the U.S. Supreme Court in Safeco Insurance Company of America v. Burr, 551 U.S. 47 (2007), to the FCA. The FCA defines “knowingly” to mean that a person “(i) has actual knowledge of the information, (ii) acts in deliberate ignorance of the truth or falsity of the information, or (iii) acts in reckless disregard of the truth or falsity of the information.” 31 U.S.C. § 3729(b)(1)(A). In Safeco, which addressed the Fair Credit Reporting Act’s nearly identical scienter requirement, the Supreme Court determined that a person who acts under an incorrect interpretation of a relevant statute or regulation does not act with “reckless disregard” if the interpretation is objectively reasonable and no authoritative guidance cautioned the person against it. Safeco, 551 U.S. at 70.
The relators in SuperValu alleged that when SuperValu sought Medicare and Medicaid reimbursements, it misrepresented its “usual and customary” drug prices. See 9 F.4th at 459. After interpreting the relevant regulations, SuperValu reported its retail cash prices as its usual and customary drug prices rather than the lower, price-matched amounts that it charged customers under its price-match discount drug program, through which SuperValu would match discounted prices of local competitors upon request from anyone purchasing. Id. The court agreed with the relator that SuperValu’s discounted prices were the correct usual and customary prices under the relevant regulations, and that SuperValu therefore should have reported those prices. The court applied the Safeco scienter standard, however, and concluded that SuperValu’s interpretation of the regulations was objectively reasonable and that there was no authoritative guidance that warned SuperValu away from its interpretation. Id. at 472. The court therefore found that SuperValu faced no liability under the FCA. Id.
The SuperValu opinion is significant for FCA defendants who are often required to interpret vague and ambiguous regulations. It provides FCA defendants with a basis to contest scienter as a matter of law, instead of leaving scienter issues for juries to decide.
In Thayer v. Planned Parenthood of the Heartland, 11 F.4th 934 (8th Cir. 2021), the Eighth Circuit explored the intersection of FCA’s scienter and falsity requirements. In that case, the relator alleged two distinct bases for FCA liability. The relator alleged that Planned Parenthood dispensed extra cycles of contraceptives without a physician’s order in violation of Iowa law and knowingly billed Iowa Medicaid Enterprise for post-abortion-related services in violation of state and federal law. The district court granted summary judgment to Planned Parenthood on both counts.
The Eighth Circuit first disposed of the claim that Planned Parenthood illegally dispensed extra cycles of contraceptives without a physician’s order, affirming that relator’s complaint was not particular enough to survive summary judgment. Id. at 940. Next, the court turned to the allegations of illegal billing for post-abortion related services. As to two of the patients, the Eighth Circuit held that the billing codes used were appropriate for the services provided, and therefore “there [was] no real dispute that Planned Parenthood did not submit a false claim for these patients.” Id. at 942. In other words, Iowa may not have intended to pay those types of claims, but there was nothing false about the information submitted. As to the remaining patients, the court noted that “to prove knowing falsity, [relator] must do more than show that the . . . billing code was wrong; she must have evidence that the defendants knowingly or recklessly cheated the government.” Id. at 943 (emphasis in original) (internal quotation marks and citation omitted). The relator contended that Planned Parenthood improperly and knowingly entered level-three billing codes for services that justified only level-two billing codes on four occasions, resulting in a minor difference in the amount reimbursed. But, in the court’s view, “a one-level difference in billing, resulting in less than a $12.00 reimbursement difference, is at most evidence of an innocent mistake or negligence, not a willful lie to cheat the government.” Id. at 944. Therefore, the court held there was no knowing falsity.
C. Fifth and Second Circuits Grapple with Scope of Public Disclosure Bar
The FCA’s public disclosure bar requires dismissal of an FCA case brought by a private plaintiff “if substantially the same allegations or transactions” forming the basis of the action have been publicly disclosed, including “in a Federal criminal, civil, or administrative hearing in which the Government or its agent is a party,” unless the relator is an “original source of the information.” 31 U.S.C. § 3730(e)(4).
In United States ex rel. Schweizer v. Canon, Inc., 9 F.4th 269 (5th Cir. 2021), the Fifth Circuit took up the issue of whether a copycat lawsuit premised on the same fundamental facts, but against a different defendant, is prohibited by the public disclosure bar. From November 2004 to December 2005, the relator worked as a General Services Administration (“GSA”) contracts manager for Océ North America Inc., a company that sold printers, copiers, and related services to the government. In 2006, the relator filed an FCA suit against Océ. She alleged that the United States was “overpaying Océ for copiers and services, and that its products were manufactured in noncompliant countries including China.” Id. at 272. Before the district court, she alleged that Océ violated the applicable “Price Reductions Clause” (by charging the government more than it did its commercial customers) and the Trade Agreements Act (by sourcing products from China and other non-TAA-compliant countries). In 2013, the district court presiding over that action approved a settlement in which Océ agreed to pay the government $1,200,000 in exchange for a release of the asserted FCA claims. In 2012—before the action became final—Océ was acquired by Canon. Id.
In 2016, the relator filed another FCA suit, this time against Canon. She alleged the same violations of the FCA as she had alleged against Océ in 2006. Canon moved to dismiss the suit, arguing that the FCA’s public disclosure bar prevented Schweizer’s claims. The district court agreed, and the Fifth Circuit affirmed the district court’s judgment on appeal. As the Fifth Circuit explained: “Schweizer’s allegations against Canon are ‘based upon’ the allegations and transactions asserted in the Océ litigation,” and “one could have produced the substance of the [Canon] complaint merely by synthesizing the public disclosures’ description of the [Océ] scheme.” Id. at 275–76 (internal quotation marks and citation omitted). The court rejected the relator’s arguments that the public disclosure bar did not apply because the companies were different, that Canon’s alleged scheme occurred at a later time, and that Canon allegedly violated additional government contracts. Id. at 276–77.
In United States ex rel. Foreman v. AECOM, 19 F.4th 85 (2d Cir. 2021) (discussed above), the Second Circuit also analyzed whether the FCA’s public disclosure bar precluded relator’s claims. The FCA disallows an action if substantially the same allegations have been “publicly disclosed” in a federal report. See 31 U.S.C. § 3730(e)(4)(A)(ii). AECOM argued that all the claims had been made available to the public through government reports presented to AECOM’s employees. The court disagreed. In interpreting the FCA’s requirement that disclosure be public, the court concluded that information does not become “public” if it is (1) not disclosed to innocent employees of the government contractor and (2) when information is disclosed, it comes with the obligation that employees keep the information confidential. Id. at 125. The court thus determined that the information had not been made public here when provided to AECOM employees through a government report. As such, the Second Circuit held that the public disclosure bar did not put an end to relator’s claim.
D. Several Courts Issue Notable Decisions on Pleading Fraud with Particularity Under Rule 9(B) in FCA Cases, and Supreme Court Invites Solicitor General’s View on Topic
In United States ex rel. Mamalakis v. Anesthetix Management LLC, 20 F.4th 295 (7th Cir. 2021), the Seventh Circuit addressed the level of specificity required for a relator’s allegations to satisfy the heightened pleading standards of Federal Rule of Civil Procedure 9(b). The relator alleged that Anesthetix fraudulently billed Medicare and Medicaid for services performed by anesthesiologists by using the code for “medically directed” services rather than the more appropriate and lower rate “medically supervised” services code. Id. at 297. After an initial dismissal without prejudice for failure to meet Rule 9(b)’s requirements, the relator filed an amended complaint including ten examples, identifying a particular procedure and anesthesiologist, and alleging the services did not qualify for payment at the medical-directed billing rate. Id.
The Seventh Circuit held that the district court’s decision to dismiss the amended complaint was “error” because the “ten examples, read in context with the other allegations in the amended complaint, provide sufficient particularity about the alleged fraudulent billing to survive dismissal.” Id. Although a relator “need not produce the invoices and accompanying representations at the outset of the suit, it is nevertheless essential to show a false statement, though this can be accomplished by including particularized factual allegations that give rise to a plausible inference of fraud.” Id. at 301 (internal quotation marks and citation omitted). The court concluded that the ten examples identifying specific procedures, anesthesiologists, and billing rates “provide[d] a particularized basis from which to plausibly infer that at least on these occasions, [defendant] presented false claims to the government.” Id. at 303. By providing such examples, the relator “injected enough precision and substantiation into his allegations of fraud to entitle him to move forward with his case.” Id.
The Sixth Circuit took a similar approach to Rule 9(b) pleading requirements in United States ex rel. Owsley v. Fazzi Associates, Inc., 16 F.4th 192 (6th Cir. 2021). There, the relator alleged “in detail, a fraudulent scheme” in which defendants allegedly fraudulently up-coded patient data and subsequently submitted inflated requests for payment to the Centers for Medicare and Medicaid Services (“CMS”). Id. at 196. But fatal to the relator’s claim was her failure “to identify any specific claims that [defendants] submitted pursuant to the scheme.” Id. (emphasis added) (internal quotation marks and citation omitted).
The court explained that the relator could have satisfied Rule 9(b)’s standard in one of two ways: identifying a “representative claim that was actually submitted to the government for payment,” or otherwise alleging facts “based on personal knowledge of billing practice” sufficient to support “a strong inference that particular identified claims were submitted to the government for payment.” Id. (emphasis in original) (internal quotation marks and citations omitted). The relator alleged “personal knowledge of billing practices” but “did not allege facts that identify any specific fraudulent claims” because she failed to identify the dates on which she reviewed patient records, the dates of any related claims for payment, or the amounts of those claims. Id. at 196–97. Acknowledging that the Rule 9(b) inquiry will turn on the facts of each particular case, the court clarified the “touchstone” consideration is “whether the complaint provides the defendant with notice of a specific representative claim that the plaintiff thinks was fraudulent.” Id. at 197. The information in the relator’s complaint failed to meet this standard and thus did not satisfy Rule 9(b). Id.
Finally, the Supreme Court invited the views of the Solicitor General on the issue of application of Rule 9(b) in FCA cases. See Johnson, et al. v. Bethany Hospice and Palliative Care LLC, No. 21-462, 2022 WL 145173, at *1 (U.S. Jan. 18, 2022). But it remains to be seen whether the Court will take up the issue. Indeed, this is not the first time parties have petitioned the Court on this issue, and the Supreme Court has rejected multiple petitions to clarify the interplay of Rule 9(b) and the FCA in recent years. See e.g., 81 U.S.L.W. 3650 (U.S. Mar. 31, 2014) (No. 12-1349). This is also not the first time the Supreme Court has asked for the views of the Solicitor General on this issue: in 2014, the Court asked for the input of the Solicitor General on whether to grant certiorari on this issue in the Fourth Circuit case of United States ex rel. Nathan v. Takeda Pharm., 707 F.3d 451 (4th Cir. 2013). After the Solicitor General urged the Court not to take up the issue, the Supreme Court denied certiorari, see Brief for the United States as Amicus Curiae Supporting Respondents, United States ex re. Nathan v. Takeda Pharm., 572 U.S. 1003 (2014); 81 U.S.L.W. 3650 (U.S. Mar. 31, 2014) (No. 12-1349).
E. Third Circuit Analyzes DOJ’s Authority to Dismiss Qui Tam FCA Cases
In October, the Third Circuit waded into the circuit split concerning the government’s statutory authority to dismiss an FCA qui tam suit. In Polansky v. Exec. Health Res. Inc., 17 F.4th 376 (3d Cir. 2021), a case in which the government initially declined to intervene in relator’s suit but then later moved to dismiss without formally intervening, the court considered two questions: (1) whether the government may move to dismiss a relator’s suit without intervening, and (2) what standard must the government meet for dismissal to be granted.
First, the court considered the government’s statutory authority to seek dismissal when it does not intervene. The court recognized the split between the D.C., Ninth, and Tenth Circuits, which read 31 U.S.C. § 3730(c) as granting authority to move for dismissal at any point in the litigation regardless of whether the government intervenes, and the Sixth and Seventh Circuits, which interpret the section to only grant authority to seek dismissal after intervention pursuant to Federal Rule of Civil Procedure 41.
In analyzing the question, the court accepted that the government may only dismiss cases where it has intervened. The court concluded that 31 U.S.C. § 3730(c)(2)(A) is not “a standalone provision that grants the Government unconditional authority to seek dismissal as a non-party.” Id. at 385. The court held that, when read in context along canons of statutory construction, 31 U.S.C. § 3730(c)(2)(A) served as a limit on relator’s rights “if—and only if—the Government proceeds with the action.” Id. The court noted that the other limitations in section 3730(c)(2), such as enabling the government to limit a relator’s ability to call witnesses where it “would interfere with … the Government’s prosecution of the case,” only make sense if the government is party to the case. Id. Furthermore, to allow the government to dismiss without intervention would limit the relator regardless of whether the government “proceeds with the action,” rendering those words superfluous. However, the Third Circuit rejected the relator’s argument that the government may only move to dismiss if it intervenes at the outset of a case. Id. at 387.
Second, the court held that, “[h]aving intervened, the Government becomes a party, and like any party, it is subject to the Federal Rules of Civil Procedure, including the rule governing Voluntary Dismissal.” Id. at 389. Recognizing that the FCA added “certain wrinkles,” the court concluded that the government must only meet the threshold requirements of Federal Rule of Civil Procedure 41(a), while also providing relator “notice and an opportunity for a hearing” under 31 U.S.C. § 3730(c)(2)(A). Id. In doing so, the Third Circuit agreed with the Seventh Circuit and expressly rejected both the Swift approach of the D.C. Circuit for being incongruous with other provisions of the FCA and relegating the Article III judge to the role of “serv[ing] . . . some donuts and coffee” as well as the Sequoia Orange approach of the Ninth Circuit for focusing solely on constitutional limits and for failing to consider the limitations of the Federal Rules of Civil Procedure on voluntary dismissal. Id. at 392–93 (quoting United States ex rel. CIMZNHCA, LLC v. UCB, Inc., 970 F.3d 835, 850 (7th Cir. 2020)).
Despite holding that the government must “intervene under § 3730(c)(3) before seeking to dismiss relator’s case,” the court ultimately construed the government’s motion to dismiss as including a motion to intervene, again adhering to the Seventh Circuit’s approach. Id. at 392. Further, the court found no abuse of discretion “[i]n light of [the] thorough examination and weighing of the interests of all the parties, and Rule 41(a)(2)’s ‘broad grant of discretion’ to shape the ‘proper’ terms of dismissal.” Id. at 393. In sum, the Third Circuit has responded to the growing circuit split over the authority of the government to dismiss whistleblower lawsuits by adopting the Seventh Circuit’s position in CIMZNHCA; the government must intervene to dismiss and the standard for dismissal should be primarily informed by Federal Rule of Civil Procedure 41.
F. Third Circuit Considers Retroactivity of Amendments to FCA
In United States ex rel. International Brotherhood of Electrical Workers Local Union No. 98 v. Fairfield Co., 5 F.4th 315 (3d Cir. 2021), the Third Circuit grappled with the question of whether (relatively) recent amendments to the FCA had retroactive effect. The Fraud Enforcement and Recovery Act of 2009 (“FERA”), Pub. L. No. 111-21, § 4, 123 Stat. 1625, amended several portions of the FCA, including eliminating the requirement that the false claim be presented to an office or employee of the United States, and removing the requirement for specific intent. Id. at 324. Fairfield challenged the purported retroactivity of the FERA amendments, claiming that should the specific intent provision remain, judgment must be in Fairfield’s favor. Id. at 329–30. The Third Circuit concluded, however, that the statute included a clear expression of retroactivity. The court also held that applying FERA’s amendments would not violate the ex post facto clause of the U.S. Constitution, because the penalties under the FCA were insufficiently punitive in nature to trigger that clause. Id. at 330–41.
After resolving the retroactivity point, the Third Circuit affirmed the district court’s orders entering judgment against Fairfield for FCA violations stemming from Fairfield’s alleged violation of the Davis Bacon Act, 40 U.S.C. § 3142, et. seq., which requires contractors performing work on federally funded construction projects to pay prevailing wages to their employees based on the classification of work performed. Id. at 323. As the court explained, a misclassification of employees may result in those individuals being underpaid, which means accompanying certifications to the government that a contractor is in compliance with the Act may be both false and material to payment for contractual performance. Id. at 323–24. Fairfield allegedly underpaid its employees as compared to the wages the Act required, but still submitted certifications to the government that it satisfied applicable wage requirements. Id. at 326–27.
G. Eleventh Circuit Addresses Novel Excessive Fines Issue
In Yates v. Pinellas Hematology & Oncology, P.A., 21 F.4th 1288 (11th Cir. 2021), the Eleventh Circuit considered whether a penalty imposed under the FCA violated the Eighth Amendment’s Excessive Fines Clause. Pinellas was a medical practice that collected and tested blood samples at its clinical laboratory. The entity purchased a second location—an oncology practice—at which it also performed testing allegedly without obtaining a new certification under the Clinical Laboratory Improvement Amendments (“CLIA”) of 1988, as required, for almost a year after the purchase. Id. at 1295. According to the qui tam suit, in which the government declined to intervene, the defendant allegedly then submitted reimbursement claims for blood tests to Medicare that miscited the CLIA certificate at its other location and other claims that misrepresented the location of service. Id. at 1296. A jury ultimately found the defendant liable for submitting 214 false claims to Medicare and found that the government sustained $755.54 in damages. Id. Under the FCA’s treble damages and statutory penalty provisions, discussed above, the district court imposed a total monetary award of $1,179,266.62 (composed of $2,266.62 in treble damages (3 x $755.54) and $1,177,000 in statutory penalties (214 x $5,500)). Id. at 1297.
The defendant challenged both the jury’s verdict and the district court’s monetary award. The Eleventh Circuit affirmed the jury’s verdict, concluding that the evidence presented at trial supported the jury’s findings that the defendant knowingly submitted materially false reimbursement claims. The court also disagreed with the defendant’s argument that the damages finding was incorrect because the government had received exactly what it paid for: 214 blood tests. The court explained that, in cases involving Medicare claims, damages are measured as the difference between what the government paid and what it would have paid if the defendant’s claims had been truthful. Id. at *1304–05. Thus, the court found that the evidence supported $755.54 in damages.
The defendant also argued the district court’s monetary award violated the Eighth Amendment’s Excessive Fines Clause. As a matter of first impression, the Eleventh Circuit considered whether the Excessive Fines Clause applies to an FCA case in which the government has declined to intervene (as was the case here). After explaining that the Eighth Amendment applies only to government imposition of fines and noting that the qui tam relator was a private citizen, the Eleventh Circuit held that an FCA monetary award is a fine for purposes of the Excessive Fines Clause, that it is the United States that imposes such a fine in a non-intervened qui tam action, and that the Eighth Amendment thus should apply to such actions. Id. at 1307. In so holding, the court focused on the fact that the government, although “not a formal party to a non-intervened qui tam action,” “remains a real party in interest,” has “significant procedural rights” in the ability to decide whether to intervene, and remains involved in non-intervened cases—namely, by preserving the ability to intervene later, receiving the vast majority of any monetary award, and maintaining control over whether a relator can dismiss a qui tam action. Id. at 1308–14. The court then weighed the proportionality of the award and found, based on the defendant’s alleged repeated violations and the fact that the award fell at the low-end of the FCA’s prescribed penalty range, that the penalty was not excessive. Id. at 1314–16.
H. DC Circuit Issues Opinion on Medicare Overpayment Rule
The D.C. Circuit issued a decision that, while not specifically adjudicating FCA liability, is likely to impact future FCA litigation.
In UnitedHealthcare Insurance Co. v. Becerra, 16 F.4th 867 (D.C. Cir. 2021), the D.C. Circuit considered a challenge by health care insurers to a rule promulgated by CMS, known as the “Overpayment Rule.” Under the Overpayment Rule, if an insurer “learns a diagnosis it submitted to CMS for payment lacks support in the beneficiary’s medical record, the insurer must refund that payment within sixty days.” Id. at 869. In the context of Medicare Advantage—private Medicare plans where members “elect to receive their health insurance through a private insurer . . . rather than directly through the government under traditional Medicare”—insurers must accurately track diagnosis and demographic information for purposes of ensuring that “risk-adjusted” payments from CMS are accurate. Id. at 869–70. For many years, the government has pursued FCA investigations and actions against insurers, alleging that they artificially inflated those “risk-adjusted” payments by manipulating diagnosis codes collected for members, and therefore owed CMS money, including under the Overpayment Rule.
Insurers brought suit seeking to invalidate the Overpayment Rule, claiming that the Overpayment Rule violated statutory provisions guaranteeing that risk-adjusted payments under Medicare Advantage would be “actuarially equivalent” to payment under traditional Medicare and employ the “same methodology.” According to insurers, the mechanisms established by the Overpayment Rule would violate these requirements by, in essence, requiring the return of “overpayments” under Medicare Advantage that departed from actuarial standards used in traditional Medicare.
The district court agreed with the insurers and struck down the rule. Id. at 880. In doing so, the district court also rejected the Overpayment Rule’s imposition of a “reasonable diligence” or negligence-type standard for identifying and reporting overpayments, which, according to the court, was inconsistent with the “knowingly” standard set forth in the FCA. Id. at 881. The D.C. Circuit, however, overturned the district court’s decision and held, as a matter of first impression, that nothing in the Overpayment Rule or applicable authority violated application of the “actuarial equivalence” requirement for the Medicare Advantage plans. Id. at 884–87. The D.C. Circuit also rejected the district court’s findings that the Rule violates the Medicare statute’s “same methodology” requirement. Id. at 891–92. The district court’s rejection of the “reasonable diligence” standard was not challenged on appeal.
It remains to be seen how the district court will apply the circuit court’s position on remand. But enactment and application of the Overpayment Rule will likely have significant consequences for potential FCA liability against health care insurers, and we will continue to watch this case closely.
V. CONCLUSION
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2022 False Claims Act Mid-Year Update, which we will publish in July 2022.
___________________________
[1] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Justice Department’s False Claims Act Settlements and Judgments Exceed $5.6 Billion in Fiscal Year 2021 (Feb. 1, 2022), https://www.justice.gov/opa/pr/justice-department-s-false-claims-act-settlements-and-judgments-exceed-56-billion-fiscal-year [hereinafter DOJ FY 2021 Recoveries Press Release].
[2] See U.S. Dep’t of Justice, Fraud Statistics Overview (Feb. 1, 2022), https://www.justice.gov/opa/press-release/file/1467811/download [hereinafter DOJ FY 2021 Stats].
[3] DOJ FY 2021 Recoveries Press Release.
[4] Id.
[5] DOJ FY 2021 Stats.
[6] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Contract Rehabilitation Therapy Providers Agree to Pay $8.4 Million to Resolve False Claims Act Allegations Relating to the Provision of Medically Unnecessary Therapy Services (July 2, 2021), https://www.justice.gov/opa/pr/contract-rehabilitation-therapy-providers-agree-pay-84-million-resolve-false-claims-act.
[7] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Northern Ohio Health System Agrees to Pay Over $21 Million to Resolve False Claims Act Allegations for Improper Payments to Referring Physicians (July 2, 2021), https://www.justice.gov/opa/pr/northern-ohio-health-system-agrees-pay-over-21-million-resolve-false-claims-act-allegations.
[8] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Medical Device Companies Alere Inc. and Alere San Diego Inc. Agree to Pay $38.75 Million to Settle False Claims Act Allegations (July 8, 2021), https://www.justice.gov/opa/pr/medical-device-companies-alere-inc-and-alere-san-diego-inc-agree-pay-3875-million-settle.
[9] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, St. Jude Agrees to Pay $27 Million for Allegedly Selling Defective Heart Devices (July 8, 2021), https://www.justice.gov/opa/pr/st-jude-agrees-pay-27-million-allegedly-selling-defective-heart-devices.
[10] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Prime Healthcare Services and Two Doctors Agree to Pay $37.5 Million to Settle Allegations of Kickbacks, Billing for a Suspended Doctor, and False Claims for Implantable Medical Hardware (July 19, 2021), https://www.justice.gov/opa/pr/prime-healthcare-services-and-two-doctors-agree-pay-375-million-settle-allegations-kickbacks.
[11] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Interface Rehab to Pay $2 Million to Resolve False Claims Act Allegations (July 23, 2021), https://www.justice.gov/opa/pr/interface-rehab-pay-2-million-resolve-false-claims-act-allegations.
[12] See Press Release, U.S. Dep’t of Justice, EEG Testing and Private Investment Companies Pay $15.3 Million to Resolve Kickback and False Billing Allegations (July 21, 2021), https://www.justice.gov/opa/pr/eeg-testing-and-private-investment-companies-pay-153-million-resolve-kickback-and-false.
[13] See Press Release, U.S. Atty’s Office for the Dist. of MT, Former Billings Rheumatologist Settles Alleged Health Care Fraud Claims for $2 Million (July 20, 2021), https://www.justice.gov/usao-mt/pr/former-billings-rheumatologist-settles-alleged-health-care-fraud-claims-2-million.
[14] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Mail-Order Diabetic Testing Supplier and Parent Company Agree to Pay $160 Million to Resolve Alleged False Claims to Medicare (Aug. 2, 2021), https://www.justice.gov/opa/pr/mail-order-diabetic-testing-supplier-and-parent-company-agree-pay-160-million-resolve-alleged.
[15] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Ascension Michigan to Pay $2.8 Million to Resolve False Claims Act Allegations (Aug. 5, 2021), https://www.justice.gov/opa/pr/ascension-michigan-pay-28-million-resolve-false-claims-act-allegations.
[16] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, County Medical Center and County Agree to Pay $11.4 Million to Resolve False Claims Act Allegations Relating to Medically Unnecessary Inpatient Admissions (Aug. 6, 2021), https://www.justice.gov/opa/pr/county-medical-center-and-county-agree-pay-114-million-resolve-false-claims-act-allegations.
[17] See Press Release, U.S. Atty’s Office for the Central Dist. of CA, Downey Company that Provides In-Home Respiratory Services Agrees to Pay Over $3.3 Million to Resolve Fraud Allegations (Aug. 25, 2021), https://www.justice.gov/usao-cdca/pr/downey-company-provides-home-respiratory-services-agrees-pay-over-33-million-resolve.
[18] See Press Release, U.S. Atty’s Office for the Dist. of DE, Connections Community Support Programs Agrees to Judgments of Over $15 Million to Resolve Health Care Fraud and Controlled Substances Allegations (Aug. 26, 2021), https://www.justice.gov/usao-de/pr/connections-community-support-programs-agrees-judgments-over-15-million-resolve-health.
[19] See Press Release, U.S. Atty’s Office for the Northern Dist. of TX, Hospital to Pay More Than $3 Million to Settle Whistleblower Suit (Aug. 27, 2021), https://www.justice.gov/usao-ndtx/pr/hospital-pay-more-3-million-settle-whistleblower-suit.
[20] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Sutter Health and Affiliates to Pay $90 Million to Settle False Claims Act Allegations of Mischarging the Medicare Advantage Program (Aug. 30, 2021), https://www.justice.gov/opa/pr/sutter-health-and-affiliates-pay-90-million-settle-false-claims-act-allegations-mischarging.
[21] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, United States Obtains $140 Million in False Claims Act Judgments Against South Carolina Pain Management Clinics, Drug Testing Laboratories and a Substance Abuse Counseling Center (Sept. 3, 2021), https://www.justice.gov/opa/pr/united-states-obtains-140-million-false-claims-act-judgments-against-south-carolina-pain.
[22] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Home Health Agency Operator BAYADA to Pay $17 Million to Resolve False Claims Act Allegations for Paying Kickback (Sept. 8, 2021), https://www.justice.gov/opa/pr/home-health-agency-operator-bayada-pay-17-million-resolve-false-claims-act-allegations-paying.
[23] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Orlando Cardiologist Pays $6.75 Million to Resolve Allegations of Performing Unnecessary Medical Procedures (Sept. 15, 2021), https://www.justice.gov/opa/pr/orlando-cardiologist-pays-675-million-resolve-allegations-performing-unnecessary-medical.
[24] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, Three Generic Pharmaceutical Companies Agree to Pay Almost Half a Billion Dollars to Resolve Alleged False Claims Act Liability, Bringing Total Payments for Price-Fixing to Nearly $900 Million (Oct. 15 2021), https://www.justice.gov/usao-edpa/pr/three-generic-pharmaceutical-companies-agree-pay-almost-half-billion-dollars-resolve.
[25] See Press Release, U.S. Atty’s Office for the Eastern Dist. of MI, USMM and VPA Pay $8.5 Million To Resolve Overpayment of Medicare Claims for Laboratory and Diagnostic Testing (Oct. 8, 2021), https://www.justice.gov/usao-edmi/pr/usmm-and-vpa-pay-85-million-resolve-overpayment-medicare-claims-laboratory-and.
[26] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Texas Pain Management Physicians Agree to Pay $3.9 Million to Resolve Allegations Relating to Unnecessary Urine Drug Testing (Oct. 22, 2021), https://www.justice.gov/opa/pr/texas-pain-management-physicians-agree-pay-39-million-resolve-allegations-relating.
[27] See Press Release, U.S. Atty’s Office for the Northern Dist. of GA, Atlanta pharmacy to pay $4.6 million to settle False Claims Act allegations regarding compound medications (Oct. 25, 2021), https://www.justice.gov/usao-ndga/pr/atlanta-pharmacy-pay-46-million-settle-false-claims-act-allegations-regarding-compound.
[28] See Press Release, U.S. Atty’s Office for MN, Physical Therapy Provider to Pay $4 Million to Resolve Alleged False Claims Act Violations (Oct. 28, 2021), https://www.justice.gov/usao-mn/pr/physical-therapy-provider-pay-4-million-resolve-alleged-false-claims-act-violations.
[29] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Medical Device Company Arthrex to Pay $16 Million to Resolve Kickback Allegations (November 8, 2021), https://www.justice.gov/opa/pr/medical-device-company-arthrex-pay-16-million-resolve-kickback-allegations.
[30] See Press Release, U.S. Atty’s Office for the N. Dist. of Ga., Anesthesia providers and outpatient surgery centers pay more than $28 million to resolve kickback and False Claims Act allegations (Nov. 9, 2021), https://www.justice.gov/usao-ndga/pr/anesthesia-providers-and-outpatient-surgery-centers-pay-more-28-million-resolve.
[31] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Kaléo Inc. Agrees to Pay $12.7 Million to Resolve Allegations of False Claims for Anti-Overdose Drug (Nov. 9, 2021), https://www.justice.gov/opa/pr/kal-o-inc-agrees-pay-127-million-resolve-allegations-false-claims-anti-overdose-drug.
[32] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, South Carolina Chiropractor Pleads Guilty and Agrees to $9 Million False Claims Act Consent Judgment (Nov. 22, 2021), https://www.justice.gov/opa/pr/south-carolina-chiropractor-pleads-guilty-and-agrees-9-million-false-claims-act-consent.
[33] See Press Release, U.S. Atty’s Office for the N. Dist. of Ga., Home health agency to pay $4.2 million to settle False Claims Act allegations (Nov. 22, 2021), https://www.justice.gov/usao-ndga/pr/home-health-agency-pay-42-million-settle-false-claims-act-allegations-0.
[34] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Crossroads Hospice Agrees to Pay $5.5 Million to Settle False Claims Act Liability (Nov. 23, 2021), https://www.justice.gov/opa/pr/crossroads-hospice-agrees-pay-55-million-settle-false-claims-act-liability.
[35] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Flower Mound Hospital to Pay $18.2 Million to Settle Federal and State False Claims Act Allegations Arising from Improper Inducements to Referring Physicians (Dec. 2, 2021), https://www.justice.gov/opa/pr/flower-mound-hospital-pay-182-million-settle-federal-and-state-false-claims-act-allegations.
[36] See Press Release, U.S. Atty’s Office for the Dist. of NJ., Pathology Practice Agrees to Pay $2.4 Million to Resolve False Claims Act Allegations (Dec. 7, 2021), https://www.justice.gov/usao-nj/pr/pathology-practice-agrees-pay-24-million-resolve-false-claims-act-allegations.
[37] See Press Release, U.S. Atty’s Office for the N. Dist. of Ga., Dr. Jeffrey M. Gallups and Entellus Medical agree to pay $4.2 million to resolve False Claims Act lawsuit alleging kick-back arrangements (Dec. 8, 2021), https://www.justice.gov/usao-ndga/pr/dr-jeffrey-m-gallups-and-entellus-medical-agree-pay-42-million-resolve-false-claims-act.
[38] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, AAR Corp. Agrees to Pay $11 Million to Settle False Claims Act Allegations on Aircraft Maintenance Contract and to Pay Penalties Assessed by the FAA (July 6, 2021), https://www.justice.gov/opa/pr/aar-corp-agrees-pay-11-million-settle-false-claims-act-allegations-aircraft-maintenance.
[39] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Devon Energy Companies Agree to Pay $6.15 Million to Settle False Claims Act Allegations for Underpaying Royalties on Gas from Federal Lands (Sept. 27, 2021), https://www.justice.gov/opa/pr/devon-energy-companies-agree-pay-615-million-settle-false-claims-act-allegations-underpaying.
[40] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Crane Company Agrees to Pay More Than $4.5 Million to Resolve False Claims Act Lawsuit for Non-Compliance with Military Specifications (Oct. 6, 2021), https://www.justice.gov/opa/pr/crane-company-agrees-pay-more-45-million-resolve-false-claims-act-lawsuit-non-compliance.
[41] See Press Release, U.S. Atty’s Office for the Western Dist. of NY, Cheektowaga Contractor Agrees To Settle False Claims Act Violations (Oct. 15, 2021), https://www.justice.gov/usao-wdny/pr/cheektowaga-contractor-agrees-settle-false-claims-act-violations.
[42] See Press Release, U.S. Atty’s Office for the Dist. of Md., Information Technology Contractor Agrees to Pay More Than $1.3 Million to Settle Federal False Claims Act Allegations of Overbilling (Dec. 21, 2021), https://www.justice.gov/usao-md/pr/information-technology-contractor-agrees-pay-more-13-million-settle-federal-false-claims.
[43] See Press Release, U.S. Atty’s Office for the W. Dist. of Tx., Maytag Aircraft Corporation Agrees to Pay $1.9 Million to Resolve Liability for 2014 Jet Fuel Spill at Fort Hood (Dec. 22, 2021), https://www.justice.gov/usao-wdtx/pr/maytag-aircraft-corporation-agrees-pay-19-million-resolve-liability-2014-jet-fuel-spill.
[44] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Florida Department of Children and Families Agrees to Pay $17.5 Million to Resolve False Claims Act Liability in Connection with SNAP Quality Control (July 12, 2021), https://www.justice.gov/opa/pr/florida-department-children-and-families-agrees-pay-175-million-resolve-false-claims-act.
[45] See Press Release, U.S. Atty’s Office for the S. Dist. of N.Y., Manhattan U.S. Attorney Settles Civil Fraud Lawsuit Against Clothing Companies and Their Former CEO for Misrepresenting the Value of Goods to Avoid Paying Customs Duties (July 28, 2021), https://www.justice.gov/usao-sdny/pr/manhattan-us-attorney-settles-civil-fraud-lawsuit-against-clothing-companies-and-their.
[46] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Tennessee Department of Human Services Agrees to Pay $6.8 Million to Resolve False Claims Act Liability in Connection with SNAP Quality Control (Aug. 5, 2021), https://www.justice.gov/opa/pr/tennessee-department-human-services-agrees-pay-68-million-resolve-false-claims-act-liability.
[47] U.S. Dep’t of Justice, Office of the Associate Attorney General Rachel Brand, Memorandum for Heads of Civil Litigating Components and United States Attorneys: Limiting Use of Agency Guidance Documents In Affirmative Civil Enforcement Cases (Jan. 25, 2018), https://www.justice.gov/file/1028756/download.
[48] U.S. Dep’t of Justice, Justice Manual § 1-20.100 (Dec. 2018), https://web.archive.org/web/20190327044939/https://www.justice.gov/jm/1-20000-limitation-use-guidance-documents-litigation.
[49] See 86 Fed. Reg. 37674 (July 16, 2021), https://www.govinfo.gov/content/pkg/FR-2021-07-16/pdf/2021-14480.pdf.
[50] U.S. Dep’t of Justice, Office of the Attorney General, Memorandum for Heads of All Department Components: Issuance and Use of Guidance Documents by the Department of Justice (July 1, 2021), https://www.justice.gov/opa/page/file/1408606/download.
[51] Id.
[52] Press Release, U.S. Dep’t of Justice, Deputy Attorney General Lisa O. Monaco Announces New Civil Cyber-Fraud Initiative (Oct. 6, 2021), https://www.justice.gov/opa/pr/deputy-attorney-general-lisa-o-monaco-announces-new-civil-cyber-fraud-initiative.
[53] Id.
[54] U.S. Dep’t of Justice, Acting Assistant Attorney General Brian M. Boynton Delivers Remarks at the Cybersecurity and Infrastructure Security Agency (CISA) Fourth Annual National Cybersecurity Summit (Oct. 13, 2021), https://www.justice.gov/opa/speech/acting-assistant-attorney-general-brian-m-boynton-delivers-remarks-cybersecurity-and.
[55] See Gibson, Dunn & Crutcher LLP, Emergency Federal Measures to Combat Coronavirus (Mar. 18, 2020), https://www.gibsondunn.com/emergency-federal-measures-to-combat-coronavirus/.
[56] American Rescue Plan Act, 2021, Pub. L. No. 117-2, 117th Cong. (2021).
[57] Press Release, U.S. Dep’t of Justice, Eastern District of California Obtains Nation’s First Civil Settlement for Fraud on Cares Act Paycheck Protection Program (Jan. 12, 2021), https://www.justice.gov/usao-edca/pr/eastern-district-california-obtains-nation-s-first-civil-settlement-fraud-cares-act.
[58] Press Release, U.S. Dep’t of Justice, Owner of Jet Charter Company Settles False Claims Act Allegations Regarding Misappropriation of Payment Protection Program Loan (Aug. 27, 2021), https://www.justice.gov/usao-sdfl/pr/owner-jet-charter-company-settles-false-claims-act-allegations-regarding.
[59] Press Release, U.S. Dep’t of Justice, COVID-19 Task Force Nets Florida Duct Cleaning Company; Settles False Claims Act Allegations Relating to Improper Paycheck Protection Program Loan (Oct. 28, 2021), https://www.justice.gov/opa/pr/covid-19-task-force-nets-florida-duct-cleaning-company-settles-false-claims-act-allegations.
[60] CARES Act, Pub. L. No. 116-136, 116th Cong. (2020), § 4018.
[61] Special Inspector Gen. for Pandemic Recovery, SIGPR Overview, https://www.sigpr.gov/about-sigpr/sigpr-overview.
[62] Special Inspector Gen. for Pandemic Recovery, Quarterly Report: Message from the Special Inspector General for Pandemic Recovery (July 30, 2021), https://www.sigpr.gov/news/quarterly-report-message-special-inspector-general-pandemic-recovery.
[63] See Press Release, U.S. Dep’t of Justice, Baltimore Woman Facing Federal Indictment for Allegedly Obtaining More Than $1.6 Million in Federal Funds Intended to Relieve Financial Distress Caused by the Covid-19 Pandemic (Dec. 15, 2021), https://www.justice.gov/usao-md/pr/baltimore-woman-facing-federal-indictment-allegedly-obtaining-more-16-million-federal (reflecting SIGPR’s involvement in action); see also Baltimore Woman Used Fake Documents To Get $1.6 Million In COVID Relief Funds, Feds Say, Balt. Sun (Dec. 15, 2021), https://www.baltimoresun.com/news/crime/bs-md-ci-cr-covid-relief-charges-20211215-wcf7ydmpozd2rm7tb77dt7o7ze-story.html.
[64] Ltr. from Sen. Charles Grassley to Hon. Merrick B. Garland (Feb. 24, 2021), https://g7x5y3i9.rocketcdn.me/wp-content/uploads/2021/03/2021-02-24-CEG-to-DOJAG-Nominee-Garland-regarding-FCA.pdf.
[65] False Claims Amendments Act of 2021, S. 2428, 117th Cong. (July 22, 2021), https://www.congress.gov/bill/117th-congress/senate-bill/2428/text/is?r=1.
[66] Press Release, Sen. Chuck Grassley, False Claims Act Amendments of 2021, https://www.grassley.senate.gov/imo/media/doc/false_claims_amendments_act_summary.pdf.
[67] False Claims Amendments Act of 2021, S. 2428, 117th Cong. (Nov. 16, 2021), https://www.congress.gov/bill/117th-congress/senate-bill/2428/text/rs?r=1.
[70] Actions Overview, False Claims Amendments Act of 2021, S. 2428, 117th Cong. (1st Sess. 2021), https://www.congress.gov/bill/117th-congress/senate-bill/2428/actions?r=1&s=1.
[71] HHS-OIG, State False Claims Act Reviews, https://oig.hhs.gov/fraud/state-false-claims-act-reviews/.
[72] Id.
[73] Id.
[74] See Ltr. from Christi A. Grimm, Principal Deputy Inspector General, to Hon. Austin Knudsen, Attorney General of Minnesota (Oct. 4, 2021), https://oig.hhs.gov/documents/false-claims-act/1003/montana2021.pdf.
[75] HHS-OIG, supra note 63.
[76] Analysis by the Legislative Reference Bureau, Wisconsin S.B. 652 (Oct. 20, 2021), https://docs.legis.wisconsin.gov/2021/related/proposals/sb652.pdf.
[77] Id.
[78] See Ltr. from Daniel R. Levinson, Inspector General, to Hon. Brad Schimel, Attorney General of Wisconsin (Dec. 28, 2016), https://oig.hhs.gov/documents/false-claims-act/276/Wisconsin-supplement.pdf.
[79] See Analysis of the Legislative Reference Bureau, supra note 76.
The following Gibson Dunn lawyers assisted in the preparation of this alert: Jonathan Phillips, John Partridge, James Zelenay, Reid Rector, Michael Dziuban, Allison Chapin, Chelsea Knudson, Becca Smith, Emma Strong, Phuntso Wangdra, Mike Ulmer, Katie King, Jabari Julien, Nick Perry, Ben Gibson, and John Turquet Bravard.
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues. Please feel free to contact the Gibson Dunn lawyer with whom you usually work, the authors, or any of the following members of the firm’s False Claims Act/Qui Tam Defense Group:
Washington, D.C.
Jonathan M. Phillips – Co-Chair, False Claims Act/Qui Tam Defense Group (+1 202-887-3546, jphillips@gibsondunn.com)
F. Joseph Warin (+1 202-887-3609, fwarin@gibsondunn.com)
Joseph D. West (+1 202-955-8658, jwest@gibsondunn.com)
Robert K. Hur (+1 202-887-3674, rhur@gibsondunn.com)
Geoffrey M. Sigler (+1 202-887-3752, gsigler@gibsondunn.com)
Lindsay M. Paulin (+1 202-887-3701, lpaulin@gibsondunn.com)
San Francisco
Winston Y. Chan – Co-Chair, False Claims Act/Qui Tam Defense Group (+1 415-393-8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415-393-8391, cstevens@gibsondunn.com)
New York
Reed Brodsky (+1 212-351-5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212-351-3850, mdenerstein@gibsondunn.com)
Alexander H. Southwell (+1 212-351-3981, asouthwell@gibsondunn.com)
Brendan Stewart (+1 212-351-6393, bstewart@gibsondunn.com)
Casey Kyung-Se Lee (+1 212-351-2419, clee@gibsondunn.com)
Denver
John D.W. Partridge (+1 303-298-5931, jpartridge@gibsondunn.com)
Robert C. Blume (+1 303-298-5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303-298-5784, mloseman@gibsondunn.com)
Ryan T. Bergsieker (+1 303-298-5774, rbergsieker@gibsondunn.com)
Reid Rector (+1 303-298-5923, rrector@gibsondunn.com)
Dallas
Robert C. Walters (+1 214-698-3114, rwalters@gibsondunn.com)
Andrew LeGrand (+1 214-698-3405, alegrand@gibsondunn.com)
Los Angeles
Nicola T. Hanna (+1 213-229-7269, nhanna@gibsondunn.com)
Timothy J. Hatch (+1 213-229-7368, thatch@gibsondunn.com)
Deborah L. Stein (+1 213-229-7164, dstein@gibsondunn.com)
James L. Zelenay Jr. (+1 213-229-7449, jzelenay@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650-849-5395, bwagner@gibsondunn.com)
© 2022 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice.
As the United States emerges from the darkest days of the COVID-19 pandemic and the Biden Administration settles in, the U.S. government and qui tam relators continue to churn out litigation and investigations under the False Claims Act (“FCA”), the government’s primary tool for combatting fraud against the federal fisc.
Six months ago, in our 2020 Year-End FCA Update, we explored what the new Biden Administration’s priorities might be and whether they would alter FCA enforcement. To date, there have been no major shifts in overarching policy, but the contours of the Biden Administration’s priorities are emerging. And, with nearly $400 million in FCA settlements in the first half of the year, more aggressive and forward-leaning FCA enforcement may well be on the horizon. Indeed, the Biden Administration forecasts that its efforts to root out COVID-19-related fraud will result in “significant cases and recoveries” under the FCA.
Meanwhile, federal courts issued several significant decisions in the first half of 2021, including important decisions exploring the use of statistical evidence in FCA cases, causation in fraudulent inducement cases, alleged “fraud on the FDA,” liability based on Anti-Kickback Statute (“AKS”) violations, the FCA’s materiality requirement, and DOJ’s discretion to dismiss qui tam cases where the government has not intervened.
Below, we begin by summarizing recent enforcement activity, then provide an overview of notable legislative and policy developments at the federal and state levels, and finally analyze significant court decisions from the past six months. Gibson Dunn’s recent publications regarding the FCA may be found on our website, including in-depth discussions of the FCA’s framework and operation, industry-specific presentations, and practical guidance to help companies avoid or limit liability under the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
I. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE FIRST HALF OF 2021
Momentum continued to build on the FCA enforcement front during 2021’s first half, as DOJ announced a number of FCA resolutions totaling more than $393 million. Although the number of resolutions demonstrated a continued high level of enforcement activity, these resolutions did not include any blockbuster settlements by historical standards; DOJ did not announce any nine-figure settlements in the first half of the year.
Below, we summarize the most notable settlements thus far in 2021, with a focus on the industries and theories of liability involved. Consistent with historical trends, a majority of FCA recoveries from enforcement actions for the first half of this year have involved health care and life sciences entities, including alleged violations of the AKS, but DOJ also announced several resolutions in the government contracting and procurement space.
A. HEALTH CARE AND LIFE SCIENCE INDUSTRIES
FCA resolutions in the health care and life sciences industries totaled more than $228 million. Consistent with historical trends, this made up the largest share of overall recoveries of any industry. Of the 27 resolutions summarized below, at least five included a Corporate Integrity Agreement.
- On January 11, a California laboratory agreed to pay $2.5 million to resolve allegations that it violated the FCA and the AKS by billing Medicare for genetic tests that were induced by kickbacks paid for referrals of the tests. A marketing company purportedly referred its clients to the laboratory for testing, and the laboratory allegedly paid a specified percentage or fixed amount of Medicare’s reimbursement for covered tests.[1]
- On February 4, a Florida company and the company’s president agreed to pay $20.3 million to settle allegations that they violated the FCA by fraudulently establishing corporations to bill for medically unnecessary durable medical equipment (“DME”) and by engaging in improper marketing practices in violation of the According to the government’s allegations, the company established dozens of front companies purporting to be DME suppliers, submitted more than $400 million in improper DME claims to Medicare and the Veterans Administration (“VA”), and bribed doctors to approve the claims even when they had not interacted with the purported beneficiaries. In addition to the settlement, the company’s president pleaded guilty to conspiracy to commit health care fraud and filing a false tax return for which she faces a maximum penalty of 13 years in federal prison. The share of the whistleblower who originally filed the action was not disclosed at the time of the settlement announcement.[2]
- On February 25, a Pennsylvania pharmacy agreed to pay $2.9 million to resolve allegations that it violated the FCA by filling prescriptions with inexpensive generic medications but billing Medicare for pricier brand-name drugs, and that it violated the Controlled Substances Act by illegally dispensing opioids and other controlled substances to individuals who did not have prescriptions.[3]
- On February 25, a global medical technology company agreed to pay $3.6 million to settle allegations that it violated the FCA by submitting improperly completed certificates of medical necessity (“CMN”) for devices that were medically unnecessary. The allegations stemmed from the company’s self-disclosure to HHS-OIG that its sales representatives at times filled out a CMN section that, under Medicare rules, must be completed by the treating physician’s office.[4]
- On March 2, a North Carolina medical equipment provider agreed to pay $20.1 million, and its individual owner agreed to pay an additional $4 million, to resolve allegations that the company violated the federal and North Carolina FCA The government alleged that the company fraudulently billed Medicaid for DME purportedly provided to individual Medicaid recipients, but the individuals never ordered or received the equipment; in some cases, the supposed recipients allegedly had been deceased for years before the submission of the claims. The U.S. government and the state of North Carolina also obtained a default judgment of $34.7 million against a sales representative of the company. In a related criminal investigation, the company was sentenced to five years of probation and ordered to pay a $2 million fine and over $10 million in restitution to the North Carolina Medicaid Program related to charges of health care fraud. The company self-reported the activity to the North Carolina Medicaid Investigations Division.[5]
- On March 2, a Virginia medical practice agreed to pay $2.1 million to resolve allegations that it violated the FCA by double-billing Medicare for treatments administered to patients. The at-issue treatment is sold in single-use vials, but some patients do not need an entire vial. In such cases, Medicare rules allow the doctor to bill as if the entire vial had been administered while discarding the leftover amount. Allegedly, the medical practice engaged in a scheme whereby it would administer a partial vial to one patient, give the remainder of the vial to a different patient, and then bill Medicare for one full vial per patient. In June 2020, the medical practice pleaded guilty to one count of criminal health care fraud related to the conduct.[6]
- On March 5, the Florida-based parent of two Ohio psychiatric hospitals and one Ohio substance abuse treatment facility agreed to pay $10.3 million to resolve allegations that they violated the FCA by billing federal health care programs for medical services that were induced by kickbacks improperly provided to patients. According to the government, the company unlawfully provided free long-distance transportation to patients to induce them to seek treatment at the company’s facilities and then submitted claims for the services it provided to those patients. The government also alleged that some of the inpatient admissions, for which the company submitted claims, were medically unnecessary. In addition to the financial settlement, the company entered into a Corporate Integrity Agreement with HHS-OIG that requires it to retain an independent reviewer for a five-year period to examine its claims to Medicare and Medicaid. The share of the whistleblower who originally filed the action was not disclosed at the time of the settlement announcement.[7]
- On March 16, two former owners of a telemarketing company agreed to collectively pay at least $4 million to settle allegations that they violated the FCA by scheming to generate referrals to pharmacies in exchange for kickbacks. The government alleged that the former owners solicited prospective patients to accept compounded drugs notwithstanding the medical necessity of such drugs, procured prescriptions for the patients, and then arranged to have those prescriptions filled at compounding pharmacies. In exchange, the former owners received a kickback from the pharmacies equal to half of the amount that TRICARE ultimately reimbursed for each prescription. Under the settlement agreement, the exact resolution amount will be determined based the sale price of certain real property that one of the former owners agreed to sell. A former employee of one pharmacy to which the telemarketing company referred prescriptions initially filed the qui tam The share of the whistleblower who originally filed the action was not publicized at the time the settlement was announced.[8]
- On March 18, a Michigan physician and his practice agreed to pay $2 million to resolve allegations that the practice violated the FCA by billing federal programs for diagnostic tests that were unnecessary or never performed. In addition to the financial settlement, the physician and his practice agreed to a Integrity Agreement with HHS-OIG that requires billing practices oversight for a three-year period. The shares of the two whistleblowers who originally filed the actions were not disclosed at the time of the settlement announcement.[9]
- On March 26, a former owner of a North Carolina diagnostic testing laboratory agreed to pay $2 million to settle allegations that he participated in kickback schemes to induce physicians to refer patients to the laboratory for medically unnecessary drug tests, leading to the submission of claims to Medicare in violation of the AKS and the FCA. According to the government, the laboratory provided benefits, including urine drug testing equipment and loans to physicians, as well as volume-based commissions and a salary to an individual for influence over two physician practices, in exchange for referrals to the laboratory for testing. On March 30, another of the laboratory’s former owners consented to an entry of final judgement requiring that he pay $4.5 million to resolve allegations that he paid kickbacks to the owner or a medical practice.[10]
- On April 1, a New York-based pharmaceutical company agreed to pay $75 million to resolve allegations that it knowingly underpaid rebates owed pursuant to the Medicaid Drug Rebate Program. The government alleged that the company had underreported the Average Manufacturer Prices (“AMPs”) for multiple drugs because it improperly subtracted service fees paid to wholesalers from the reported AMPs and excluded additional value the company received under contractual price-appreciation provisions with the wholesalers. According to the government, the underreported AMPs resulted in underpaid quarterly rebates to states and, relatedly, caused overcharges to the United States for the government’s Medicaid program payments to the states.[11] The company will pay approximately $41 million, plus interest, to the United States and the remainder to states participating in the settlement. The settlement stemmed from a qui tam lawsuit, which the whistleblower pursued after the government declined to intervene. The whistleblower’s share was not announced with the settlement.
- On April 8, an urgent-care provider network in South Carolina and its management company agreed to pay $22.5 million to resolve allegations that the management company falsely certified that network health care providers credentialed to bill Medicaid, Medicare, and TRICARE had performed various procedures, when non-credentialed providers actually performed those services. The companies also entered into a five-year Corporate Integrity Agreement with HHS-OIG and DCIS that requires the management company to retain an independent review organization to review its claims.[12] The share of the whistleblowers who originally filed the action was not announced with the settlement.
- On April 20, a network of three specialty health care providers in Massachusetts agreed to pay $2.6 million to resolve allegations that they improperly billed Medicare and Massachusetts’ Medicaid program for certain office visits while also billing for procedures performed at the office visits, allowing the providers to obtain reimbursements to which they were not entitled under the circumstances. The whistleblower who originally filed the action will receive 15% of the recovery.[13]
- On April 21, a Tennessee-based network of pain-management clinics, its four majority owners, and a former executive agreed to pay $4.1 million to settle allegations involving the submission of false claims for medically unnecessary or non-reimbursable treatments, testing, and drugs to federal health care programs, as well as for services and testing that were not actually performed. The settlement also resolved common-law claims of fraud, payment by mistake, and unjust enrichment. With the settlement, the government agreed to dismiss its underlying civil action against all the parties except the network’s former CEO, who was convicted of health care fraud in 2019. The allegations originally stemmed from qui tam lawsuits, pursuant to which the whistleblowers will receive $610,685.[14]
- On April 30, a health care software developer in Florida agreed to pay $3.8 million to resolve allegations that it used its marketing referral program for electronic health records products to pay unlawful kickbacks to generate sales. The government alleged that the referral program financially incentivized existing clients to recommend the developer’s products and barred program participants from providing negative product information to prospective clients, without the prospective clients’ knowledge of the arrangement. The government also asserted that the kickback payments rendered false the claims the company submitted under Medicare and Medicaid Meaningful Use Programs and the Merit-Based Incentive Payment System. The whistleblower who originally filed the action will receive approximately $800,000 in connection with the settlement.[15]
- On May 3, a neurosurgeon in South Dakota, as well as two affiliated medical device distributors owned by the doctor, agreed to pay $4.4 million to resolve allegations that the doctor accepted illegal payments to use certain medical devices and knowingly submitted claims for medically unnecessary surgeries. The doctor allegedly requested and received kickbacks, in the form of meals and alcohol, from a medical device manufacturer through a restaurant that the doctor owned with his wife. The doctor also allegedly knowingly submitted false claims for medically unnecessary procedures using medical devices in which he had a financial interest. The two medical device distributors agreed to pay an additional $100,000 to resolve claims that they violated the Centers for Medicare & Medicaid Services’ (“CMS”) Open Payments Program when the distributors failed to report to the CMS the doctor’s ownership interests and payments made to him. The settlement precludes each of the defendants from participating in federal health care programs for a period of six years. The whistleblowers who originally filed the action will receive $880,000 in connection with the settlement.[16]
- On May 4, a Delaware-based pharmaceutical manufacturer agreed to pay $12.6 million to resolve allegations that it used a third-party foundation to cover the copays of Medicare and TRICARE patients taking its myelofibrosis drug. The government alleged that the manufacturer improperly induced patients to purchase its drugs after pressuring the foundation to use funds donated by the manufacturer for patient copays and help ineligible patients complete financial assistance applications to the fund. The whistleblower who originally filed the action will receive approximately $3.59 million of the recovery.[17]
- On May 5, an Arizona hospital, operated by one of the largest health care systems in the United States, and a neurosurgery provider on the hospital’s campus agreed to pay $10 million to resolve allegations that they billed Medicare for concurrent, overlapping surgeries in violation of regulations and reimbursement policies. The neurosurgery provider contemporaneously entered into a five-year Corporate Integrity Agreement with HHS-OIG that requires the provider to maintain compliance and risk-assessment programs and hire an independent review organization to annually review its claims. The share of the recovery the whistleblower who originally filed the action was not announced with the settlement.[18]
- On May 10, a private university in Florida agreed to pay $22 million to resolve claims related to its laboratory and off-campus, hospital-based facilities. The government alleged that the university billed federal health care programs for medically unnecessary laboratory tests for kidney transplant patients, submitted inflated reimbursement claims for pre-transplant laboratory testing in violation of regulations limiting above-cost reimbursements for tests performed by a provider’s related entity, and knowingly failed to provide required notice to beneficiaries regarding the cost of receiving services at hospital facilities rather than physician offices. Contemporaneous with the settlement, the university entered into a five-year Corporate Integrity Agreement with HHS-OIG, which requires the university to establish compliance, risk-assessment, and internal-review programs. The share of the whistleblower who originally filed the three underlying qui tam lawsuits was not disclosed at the time of settlement.[19]
- On May 11, a national pharmacy-services provider based in Texas agreed to pay $2.8 million to resolve a number of alleged violations under the Controlled Substances Act and FCA. The settlement also resolved allegations that the provider dispensed opioids and other controlled substances without valid prescriptions, submitted false claims for invalid emergency prescriptions to Medicare, and billed Medicare for claims that had already been reimbursed. The share of the whistleblower who originally filed the action was not announced with the settlement.[20]
- On May 14, two Texas-based dentists, as well as their affiliated practices and dental management companies, agreed to pay $3.1 million to resolve allegations that they knowingly billed Medicaid for services not rendered or falsely identified who provided those services. The share of the whistleblowers who originally filed the action was not announced with the settlement.[21]
- On May 19, a French medical device manufacturer and its American affiliate agreed to pay $2 million to resolve allegations that they violated the AKS, FCA, and the Open Payments Program’s requirements. The government alleged the manufacturer provided items of value—such as meals, entertainment, and travel expenses—to U.S.-based doctors attending a conference in France to induce purchases of their spinal devices and failed to fully report the physician-entertainment expenses as part of the Open Payments Program. The share of the whistleblower who originally filed the action was not announced with the settlement.[22]
- On May 21, an Atlanta-based chain of nursing facilities agreed to pay $11.2 million to resolve allegations that it billed Medicare for medically unreasonable, unnecessary, and unskilled rehabilitation therapy services, and that it billed both Medicare and Medicaid for substandard or “worthless” skilled-nursing services after allegedly failing to have a sufficient number of skilled nursing staff to care for the residents. The settlement also resolved allegations that the chain submitted false claims to Medicaid for coinsurance amounts for beneficiaries eligible for both Medicare and Medicaid. Contemporaneously, the chain entered into a five-year Corporate Integrity Agreement with HHS-OIG that requires an independent organization to annually review patient stays and associated claims as well as an independent monitor to review resident-care quality. The settlement resolves several qui tam suits; the whistleblowers’ share of the recovery was not announced with the settlement.[23]
- On May 25, a dental-clinic system in New York agreed to pay $2.7 million to resolve allegations that it submitted false claims to Medicaid for dental services performed with improperly sterilized equipment. The share of the whistleblower who originally filed the action was not announced with the settlement.[24]
- On June 8, a Texas-based chiropractor and her medical group agreed to pay $2.6 million to resolve allegations that the chiropractor improperly billed Medicare and TRICARE programs for the implantation of neurostimulator electrodes despite not performing such surgeries. In addition to the settlement, the chiropractor and affiliated medical entities agreed to a 10-year period of exclusion from participation in any federal health care program.[25]
- On June 28, a surgery center and its affiliated outpatient surgery provider agreed to pay $3.4 million to resolve allegations that the companies submitted claims for kidney stone procedures that were not medically justified and also engaged in a kickback scheme. One of the surgery centers allegedly submitted claims for certain kidney stone procedures for Medicare and TRICARE patients that were not medically necessary. Further, a physician and the two companies allegedly engaged in a kickback arrangement in which the physician performed the kidney stone procedures in exchange for per-procedure payments at the surgery center, which the surgery center then billed to Medicare and TRICARE. The settlement resulted from a qui tam lawsuit, and the whistleblower will receive $748,000 of the settlement proceeds. In November 2020, the estate of the physician also paid the U.S. government $1.75 million to resolve claims related to his participation in the conduct.[26]
B. GOVERNMENT CONTRACTING AND PROCUREMENT
Settlement amounts to resolve liability under the FCA in the government contracting and procurement space totaled more than $165 million in the first half of 2021.
- On January 8, a Connecticut electrical contractor agreed to pay $3.2 million to settle allegations that it violated the FCA in connection with public construction contracts principally funded by the U.S. Department of Transportation. Under the terms of the contracts, the contractor was required to subcontract a portion of the work to Disadvantaged Business Enterprises (“DBE”). The government alleged that the contractor fraudulently misrepresented that a DBE had performed work as a subcontractor, when in fact the work in question was performed by the electrical contractor’s own employees. As part of the settlement, the contractor agreed to enter a monitoring agreement with the Federal Transit Administration.[27]
- On January 12, a Washington aerospace contractor agreed to pay $25 million to resolve allegations that it submitted materially false cost and pricing data in relation to military contracts, in violation of the FCA. The contractor entered into contracts to supply Unmanned Aerial Vehicles (“UAVs”) to the military. The proposals submitted by the contractor incorporated cost and pricing data that assumed new parts would be used in building the UAVs, but the government alleged that the contractor instead used recycled, refurbished, reconditioned, or reconfigured parts. The whistleblower who originally filed the qui tam lawsuit will receive $4.625 million of the settlement amount.[28]
- On February 17, a subsidiary of a French civil engineering company agreed to pay $3.9 million to resolve allegations that it violated the FCA by knowingly using contractually noncompliant concrete in the construction of an overseas U.S. military airfield. In addition to the civil settlement, the company agreed to enter into a separate DPA under which the company admitted to the underlying facts and accepted responsibility for a one-count information for conspiracy to commit wire fraud, and agreed to pay a monetary penalty of more than $12.5 million. The civil settlement credited approximately $1.95 million of the DPA payment.[29]
- On February 19, a Virginia company agreed to pay more than $6 million to settle allegations that its predecessor company, an information technology contractor, violated the FCA by overbilling the Department of Homeland Security (“DHS”) for work performed by its employees. The contractor allegedly used underqualified personnel to perform services and knowingly billed DHS at higher rates meant for more qualified personnel.[30]
- On February 26, a U.S.-based airline agreed to pay $49 million to resolve criminal charges and civil claims that it provided fraudulent data to the U.S. Postal Service (“USPS”) in connection with a contract to deliver mail internationally on behalf of U.S.P.S. Under the airline’s contracts with USPS, it was required to provide bar code scans of mail containers when it took possession of them and again when it delivered them to intended recipients; the airline was entitled to payment only if accurate scans were provided and the mail was timely delivered. According to the government, the airline submitted automated scans that did not correspond to the actual movement of the mail, and thus it was not entitled to payment. The airline admitted that it concealed problems related to mail movement and scanning that would have subjected it to penalties under the contracts. The airline agreed to pay nearly $32.2 million to resolve civil allegations that it violated the FCA, and the airline also entered into a criminal non-prosecution agreement and agreed to pay an additional $17.3 million in criminal penalties and disgorgement. The airline also agreed to continued cooperation with the DOJ Criminal Division’s Fraud Section. The airline further agreed to strengthen its compliance program and agreed to reporting requirements, including annual reports to DOJ.[31]
- On March 1, the subsidiary of a multinational software engineering and support company agreed to pay $2.2 million to settle allegations that it violated the FCA by failing to pay required administrative fees pursuant to contracts it signed with the U.S. General Services Administration, and that it violated the FCA by failing to provide contracted discounts and not meeting contractual requirements regarding the educational and experiential qualifications of its staff.[32]
- On March 19, a New York-based nongovernmental organization (“NGO”) agreed to pay $6.9 million to settle allegations that it violated the FCA in relation to programming funded by the U.S. Agency for International Development (“USAID”). The NGO received USAID funding to provide humanitarian assistance to refugees in Syria. According to the government, the NGO’s staff participated in a collusion and kickback scheme with a foreign supplier to rig bids for goods and services contracts used in its humanitarian relief efforts. The government alleged that this conduct led to the procurement of goods at unreasonably high prices, which were then invoiced to USAID.[33]
- On April 29, a California-based manufacturer agreed to pay $5.6 million to resolve allegations that it falsely certified the origin of materials and the manufacturing location of items produced under a contract with the Government of Israel, which was funded by the U.S. Defense Security Cooperation Agreement Agency. To be eligible for foreign procurement grant funds, the materials must be sourced and manufactured in the United States by domestic companies. As related to items manufactured under the DSCA-funded contract with the Government of Israel, the government alleged that the manufacturer knowingly submitted false certifications that Chinese-sourced materials were produced in the United States and that manufacturing had occurred in the United States, when the company had in fact contracted with a Mexican maquiladora. The whistleblowers who filed the action will receive 17% of the settlement.[34]
- On May 27, an Illinois-based military manufacturer agreed to pay $50 million to resolve allegations that it fraudulently induced the U.S. Marine Corps to enter into a contract modification at inflated prices for components of armored vehicles. The government alleged that the manufacturer knowingly created and submitted fraudulent sales invoices for sales that never occurred to justify the contract’s inflated prices. The whistleblower who filed the action will receive approximately $11.1 million of the settlement.[35]
- On June 3, a Washington subsidiary of a Colorado-based environmental cleanup and remediation company paid approximately $3 million to resolve allegations that it submitted fraudulent small-business subcontractor reports. The company had entered into a government contract that required it to make efforts to award small businesses a percentage of its subcontracts and regularly report its progress; the contract provided fee-based incentives for its subcontracting successes and imposed monetary penalties if these goals were missed in bad faith. The government alleged that the company falsely represented the status of two businesses awarded subcontracts to claim credit for small-business subcontractors under the contract. The whistleblowers who originally filed the action will receive approximately $865,900 of the settlement.[36]
- On June 10, a national car-rental group headquartered in New Jersey agreed to pay $10.1 million to resolve allegations that it submitted false claims under an agreement managed by the Department of Defense Travel Management Office for unallowable supplemental charges to car rentals, such as collision-damage waiver insurance, supplemental liability coverage, personal-effects coverage, and late turn-in fees. Additionally, the government alleged that some of the fees charged were already included in the government rental rate.[37]
- On June 25, a multinational telecommunications and Internet service provider company agreed to pay more than $12.7 million to resolve allegations that the company violated the FCA in numerous ways. Former officials of the company allegedly accepted kickbacks in return for favorable treatment for subcontractors related to government contracts. The company also allegedly improperly obtained protected competitor bid information related to a government contract to gain a bidding advantage. Further, the company allegedly misstated its compliance with woman-owned small business subcontracting requirements under a contract with the Department of Homeland Security. The settlement resolves claims under the FCA, the Anti-Kickback Act, and the Procurement Integrity Act. The share of the whistleblower who originally filed the action was not disclosed at the time of the settlement announcement.[38]
- On June 30, a government contractor agreed to pay $4.3 million to settle allegations that three of its former executives accepted kickbacks from a subcontractor in exchange for awarding subcontracts for government contracts. A former executive allegedly instructed a subcontractor to mark up the cost of the subcontractor’s services provided to the contractor, and instructed the subcontractor to divide the proceeds between the subcontractor, the former executive, and two other former executives in exchange for awarding the subcontracts to the subcontractor.[39]
II. POLICY AND LEGISLATIVE DEVELOPMENTS
A. COVID-19-RELATED DEVELOPMENTS
During the first half of 2021, DOJ has maintained its focus on COVID-19-related fraud. In a February 17, 2021 speech at the Federal Bar Association Qui Tam Conference, Acting Assistant Attorney General Brian M. Boynton outlined the Civil Division’s key enforcement priorities and placed pandemic-related fraud at the top of the list.[40] Acting AAG Boynton described ongoing efforts by DOJ and its agency partners to “identify, monitor, and investigate the misuse of critical pandemic relief monies,” and also expressed confidence that DOJ’s devotion of resources to this effort will be worthwhile: “The vast majority of the funds distributed under [pandemic relief] programs have gone to eligible recipients. Unfortunately, however, some individuals an1 businesses applied for—and received—payments to which they were not entitled.”[42]
In his remarks, Acting AAG Boynton highlighted DOJ’s first civil settlement under the PPP.[42] The settlement was small (only $100,000), but marked the first such settlement related to COVID PPP funds and resolved claims a company had violated the FCA and the Financial Institutions Reform, Recovery and Enforcement Act (FIRREA) based on allegations the company “made false statements to federally insured banks that [it] was not in bankruptcy in order to influence those banks to approve, and the Small Business Administration (SBA) to guarantee” a PPP loan.[43] And while the PPP-related settlement did not involve a qui tam relator, in March, DOJ confirmed what many in the defense bar have long known or suspected—namely that “whistleblower complaints have been on the rise” during the COVID-19 pandemic.[44]
The other priorities Acting AAG Boynton outlined in his February speech also reveal that DOJ views pandemic-related fraud as extending beyond relief programs implemented during the pandemic. For example, in discussing DOJ’s continued focus on the opioid crisis, Acting AAG Boynton characterized the crisis as “not new, but . . . exacerbated by the pandemic.”[45] Similarly, he attributed DOJ’s “continued focus on telehealth schemes” in part to “the expansion of telehealth during the pandemic.”[46] These remarks make clear that DOJ has not lost sight of pre-pandemic enforcement priorities, in addition to focusing on fraud tied to government programs that are themselves creatures of the pandemic.
B. CONTENDING WITH THE LEGACY OF THE GRANSTON MEMO
Under the Trump administration, DOJ took prominent steps to assert DOJ’s control of FCA lawsuits. Specifically, on January 10, 2018, Michael Granston, the then-Director of the Fraud Section of DOJ’s Civil Division, issued a memorandum directing government lawyers evaluating a recommendation to decline intervention in a qui tam FCA action to “consider whether the government’s interests are served . . . by seeking dismissal pursuant to 31 U.S.C. § 3730(c)(2)(A).”[47] That policy was then formally incorporated into the Justice Manual. After that, DOJ became noticeably more willing to seek dismissal of certain FCA cases.
Thus far in 2021, the Biden Administration has not signaled whether it plans to scale back DOJ’s efforts to dismiss certain qui tam suits. Nor has the Administration disavowed the principles outlined in the Granston Memo or Justice Manual. However, statements by DOJ officials in the last six months suggest that DOJ may be adapting its approach to qui tam enforcement by enhancing the government’s own ability to identify and pursue FCA violations without prompting from relators. In his February speech, Acting AAG Boynton stated explicitly that observers can “expect the Civil Division to continue to expand its own efforts to identify potential fraudsters, including its reliance on various types of data analysis.”[48] He went on to discuss “sophisticated analyses of Medicare data” by DOJ “to uncover potential fraud schemes that have not been identified by whistleblower suits, as well as to help analyze and support the allegations that we do receive from such suits.”[49]
While the Biden Administration DOJ explores its options, there has been continued criticism by Senator Chuck Grassley (R-IA) of DOJ’s use of its dismissal authority under the FCA. A week after Acting AAG Boynton’s remarks, Senator Grassley wrote to then-Attorney General Nominee Merrick Garland that “it is up to the courts, through a hearing, to determine whether or not a [qui tam] case lacks merit.”[50] According to Senator Grassley, “[t]he Justice Department is not, and cannot be, the judge, jury, and executioner of a relator’s claim.”[51] Senator Grassley asserted that he is “working with a cadre of bipartisan Senate colleagues to draft legislation that will further strengthen and improve the False Claims Act.”[52]
While the degree of DOJ involvement in this legislative effort—and the extent to which it addresses DOJ’s dismissal authority—remains to be seen, the balance between DOJ-pursued FCA cases and relator-driven matters may shift. On one level, increased leveraging of data analytics could result in less reliance on relators overall, and therefore fewer situations in which DOJ attempts to exercise its dismissal authority and risks making bad law. On another, an increase in the volume and sophistication of DOJ’s data analyses of cases that do involve relators could better position DOJ to make merits-based arguments in favor of dismissal in the event that judicial scrutiny of those decisions ratchets up.
C. A PIVOT AWAY FROM THE BRAND MEMO?
In January 2018, then-Associate Attorney General (the third-ranking position at DOJ) Rachel Brand issued a memorandum titled “Limiting Use of Agency Guidance Documents In Affirmative Civil Enforcement Cases.”[53] The so-called “Brand Memo” expressly asserted that “[g]uidance documents” issued without notice-and-comment rulemaking “cannot create binding requirements that do not already exist by statute or regulation.”[54] Therefore, the Brand Memo stated that DOJ “may not use compliance with guidance documents as a basis for proving violations of applicable law in [affirmative civil enforcement] cases.”[55] The Brand Memo also explained that DOJ “should not treat a party’s noncompliance with an agency guidance document as presumptively or conclusively establishing that the party violated the applicable statute or regulation.”[56] Despite its brevity—under two pages—the Brand Memo represented a substantial policy change for civil enforcement, especially for the FCA. In December 2018, DOJ issued new section 1-20.000 of the Justice Manual, “Limitation on Use of Guidance Documents in Litigation,” which incorporated the Brand Memo and explained that, with some important caveats—such as the use of “awareness of [a] guidance document” as evidence of scienter—DOJ “should not treat a party’s noncompliance with a guidance document as itself a violation of applicable statutes or regulations.”[57]
Under the Biden Administration, DOJ may marginalize the Brand Memo. On the day he was inaugurated, President Biden issued an executive order that signaled an expected shift from the Trump Administration’s skepticism of agencies toward greater deference to agency expertise and guidance. Executive Order 13992 revoked six Trump executive orders relating to agency regulation.[58] This included revoking Trump’s Executive Order 13891 (“Promoting the Rule of Law Through Improved Agency Guidance Documents”), which required that “agencies treat guidance documents as non-binding both in law and in practice, except as incorporated into a contract” and stated as a matter of executive policy that “[a]gencies may impose legally binding requirements on the public only through regulations and on parties on a case-by-case basis through adjudications.”[59]
President Biden’s order noted that “executive departments and agencies . . . must be equipped with the flexibility to use robust regulatory action to address national priorities,” which include addressing the “coronavirus disease 2019 (COVID-19) pandemic, economic recovery, racial justice, and climate change” (emphasis added).[60] Although Executive Order 13992 does not expressly refer to DOJ’s civil enforcement or the FCA, the Order may foster a climate in which DOJ is more willing to use sub-regulatory guidance as the basis for FCA allegations. Such a change would both allow for broader FCA enforcement and signal support for the expertise of agencies in promulgating external-facing guidance. Likewise, as companies continue to adapt to DOJ’s efforts to root out fraud in government programs, a renewed focus on agency guidance could change the risk calculus built into corporate compliance programs and internal investigation efforts.
D. STATE LEGISLATIVE DEVELOPMENTS
The federal government provides incentives for states to conform their false claims statutes to the federal FCA. In particular, HHS-OIG grants “a 10-percentage-point increase” in a state’s share of any recoveries under the relevant laws to any state that obtains HHS-OIG approval for its false claims statute.[61] Such approval requires that the statute in question, among other requirements, “contain provisions that are at least as effective in rewarding and facilitating qui tam actions for false or fraudulent claims as those described in the [federal] FCA.”[62] The statute is also required to contain a 60-day sealing provision and “a civil penalty that is not less than the amount of the civil penalty authorized under the [federal] FCA.”[63] The total number of states with approved statutes is now twenty-two, with Minnesota having obtained approval on May 27, 2021.[64] That leaves seven states—Florida, Louisiana, Michigan, New Hampshire, New Jersey, New Mexico, and Wisconsin—with false claims statutes listed by HHS-OIG as “not approved.”[65]
There have been several other notable developments in state-level false claims legislation in the first half of this year.
- In Montana, the legislature passed a law in April that changes the order of priority according to which damages and penalties not paid to qui tam relators are to be disbursed to affected government entities.[66] The statute previously provided that the affected government entity’s general fund would receive the balance of such monies; under the new law, the monies “must be distributed first to fully reimburse any losses suffered by the governmental entity as a result of the defendant’s actions,” with the remainder then going to the entity’s general fund.[67]
- In Arkansas, which has a false claims statute specific to its Medicaid program, the General Assembly recently approved a bill granting the state’s Attorney General the ability to intervene in cases brought in federal court under the federal FCA that implicate Arkansas Medicaid funds.[68]
- In California, the legislature introduced a bill that would (among other things) levy a 1% annual “wealth tax” on any resident with a net worth of over $50 million (or $25 million in the case of a married taxpayer who files a separate return).[69] The bill contains a provision subjecting false claims and records concerning the wealth tax to liability under California’s false claims statute.[70]
III. CASE LAW DEVELOPMENTS
The first half of 2021 saw a number of notable federal appellate court decisions, which we have summarized below.
A. D.C. CIRCUIT EXPLORES CAUSATION IN FCA CASES PREMISED ON “FRAUDULENT INDUCEMENT” THEORY
In United States ex rel. Cimino v. International Business Machines Corp., the D.C. Circuit issued an important opinion exploring the contours of the “fraudulent inducement” theory of FCA liability, under which an initial fraud during procurement of a contract allegedly results in liability for all claims submitted to the federal government under that contract. No. 19-7139, 2021 WL 2799946 (D.C. Cir. July 6, 2021). In its decision, the D.C. Circuit imposed important limits on the fraudulent inducement theory by requiring a relator to plead (and ultimately prove) but-for causation.
The Cimino case involved allegations that IBM had “violated the FCA by (1) using a false audit to fraudulently induce the IRS to enter into a $265 million license agreement for software the IRS did not want or need, and (2) presenting false claims for payment for software that the IRS never received.” Id. at *1. In evaluating what it deemed an issue of first impression, the D.C. Circuit undertook an in-depth review of fraudulent inducement cases under the FCA, and the Supreme Court’s most recent opinions in FCA cases, to conclude that “a successful claim for fraudulent inducement requires demonstrating that a defendant’s fraud caused the government to enter a contract that later results in a request for payment.” Id. at *4. The court explained that the critical question for “liability under the FCA for fraudulent inducement must turn on whether the fraud caused the government to contract.” Id. Turning to what standard of causation applied, the court rejected a lesser standard urged by the Relator and instead held that the FCA requires the relator or government “to allege actual cause under the but-for test,” which required the relator in Cimino to “provide sufficient facts for the court to draw a reasonable inference that IBM’s false audit caused the IRS to enter the license agreement.” Id. at *6 (emphasis added). Notably, the court also rejected relator’s argument that causation was encompassed within the FCA’s materiality requirement, and did not need to be pled separately. The court instead recognized that “a plaintiff must plead both causation and materiality,” id. at *7, and that those are “separate elements that we cannot conflate,” id. at *5.
Applying these standards, the D.C. Circuit concluded that the Relator had met his pleading burden in this particular case. But by setting forth this rigorous analysis of the causation and materiality requirements under the FCA in fraudulent inducement cases, the court also charted a course for defendants facing liability under similar circumstances. Where a relator does not plead that a defendants conduct actually caused the government to enter into the underlying contract, a fraudulent inducement theory should not be able to move forward.
Turning to relator’s second theory, the court did dismiss certain claims under Federal Rule of Civil Procedure 9(b) (which requires pleading fraud claims with particularity). Applying a strict form of Rule 9(b), the court concluded that the relator failed to plead certain claims with sufficient particularity because he did not plead “when the false claims were presented and who presented those claims.” Id. at *9.
Finally, in a concurrence, Circuit Judge Rao went a step further and questioned whether fraudulent inducement is even a valid theory under the FCA. Applying a textualist framework, he argued that “[t]he text of the FCA does not readily suggest liability for fraudulent inducement as a separate cause of action.” Id. at *9 (Rao, J., concurring). The concurrence explained that courts across the country have long accepted fraudulent inducement theories based largely on an eighty-year-old Supreme Court FCA decision in United States ex rel. Marcus v. Hess, 317 U.S. 537 (1943), superseded by statute on other grounds, Act of Dec. 23, 1943, ch. 377, 57 Stat. 608, 609. See Cimino, 2021 WL 2799946, at *10. But Judge Rao said that decision is “hardly a model of clarity regarding the existence of a fraudulent inducement cause of action,” and suggested that a “reconsideration of a fraudulent inducement cause of action may be warranted because it exists in some tension with recent Supreme Court decisions” that emphasize the text of the statute over its purpose. Id. at *11. We will be watching carefully to see if other courts take up this project of reconsideration.
B. ELEVENTH CIRCUIT DECIDES THAT QUI TAM CHALLENGE MIGHT SURVIVE SUMMARY JUDGMENT DESPITE GOVERNMENT’S CONTINUED PAYMENT
In Universal Health Services v. United States ex rel. Escobar, 136 S. Ct. 1989 (2016), the Supreme Court directed the district courts to scrutinize whether plaintiffs have alleged facts sufficient to satisfy the “rigorous” and “demanding” materiality standard the FCA imposes. The Supreme Court also emphasized that the government’s decision to continue paying claims, despite knowledge of an alleged deficiency with those claims, is “very strong evidence” that those issues are not material for purposes of the FCA. Since then, the federal courts have grappled with the impact of these instructions.
Earlier this year, the Eleventh Circuit addressed this issue in United States ex. rel. Bibby v. Mortgage Investors Corp., 987 F.3d 1340 (11th Cir. 2021), cert. denied sub nom. Mortg. Invs. Corp. v. United States ex rel. Bibby, No. 20-1463, 2021 WL 1951877, at *1 (U.S. May 17, 2021). In Bibby, the relators alleged that lenders were charging fees prohibited by the U.S. Department of Veterans Affairs (“VA”) regulations (attorneys’ fees) while certifying that they charged only permissible fees (title examination and insurance fees) by bundling them together. Id. at 1343-45. The district court granted summary judgment for the lender defendants on materiality grounds in light of the fact that the government continued to pay the claims after being on notice of the alleged issue. See id. at 1346
The Eleventh Circuit reversed, holding that genuine issues of material fact precluded summary judgment. Id. There was no dispute that the VA was aware of the lenders’ noncompliance with fee requirements, so the issue of material fact was how the VA reacted to the knowledge that the lenders were charging prohibited fees. Id. at 1349-50. The court acknowledged that the government’s payment decision is typically relevant to the materiality inquiry, but asserted that the relevance of that fact “var[ies] depending on the circumstances.” Id. at 1350. In this case, the Eleventh Circuit found it significant that “[o]nce the VA issues guaranties, it is required by law to honor those guaranties” and pay holders in due course, “regardless of any fraud by the original lender.” Id.
Having decided to “divorce [its] analysis from a strict focus on the government’s payment decision,” the court “s[aw] no reason to limit [its] view only to the VA’s issuance of guaranties.” Id. at 1351. Instead, the court reviewed “the VA’s behavior holistically” and found evidence of materiality in a VA circular sent to lenders reminding them of the applicable fee regulations, as well as the VA’s implementation of “more frequent and more rigorous audits.” Id. Although the VA neither revoked payment on guaranties of loans with purportedly fraudulent fees nor prohibited those lenders from participating in the program, the court determined that those facts did not answer the materiality question on their own. See id. at 1352. In ultimately concluding that the question of materiality in this case was one for the fact finder, the panel again emphasized that “the materiality test is holistic, with no single element—including the government’s knowledge and its enforcement action—being dispositive.” Id.
The Supreme Court denied the petition for writ of certiorari on May 17, 2021. Bibby, 2021 WL 1951877, at *1. The Eleventh Circuit court’s decision in Bibby stands as an indicator that the meaning of Escobar continues to evolve.
C. NINTH AND ELEVENTH CIRCUITS LIMIT USE OF STATISTICAL EVIDENCE AS SUFFICIENT TO MEET BOTH PLAUSIBILITY AND PARTICULARITY REQUIREMENTS OF FCA PLEADINGS
Courts have continued to clarify pleading requirements for FCA claims under Federal Rules of Civil Procedure 8(a) and 9(b).
In Integra Med Analytics LLC v. Providence Health & Services, No. 19-56367, 2021 WL 1233378, at *1 (9th Cir. Mar. 31, 2021), a Ninth Circuit panel held that Integra’s statistical analysis of publicly available data—allegedly demonstrating that Providence Health submitted Medicare claims “with higher-paying diagnosis codes” than other comparable institutions—was not enough to plead falsity when Integra had failed to rule out an “obvious alternative explanation” and therefore failed to meet the Rule 8(a) requirement for pleading a plausible claim for relief. Id. at *1, *3 (citing Bell Atl. Corp. v. Twombly, 550 U.S. 544, 557 (2007)).
The court noted that Integra, in its pleading, had not ruled out an alternative explanation for why Providence Health’s claim submissions included more Medicare reimbursement codes—in this case, major complication or comorbidity (“MCC”) codes—than other institutions: namely that Providence, with the assistance of third-party billing consultant JATA aimed at improving its Medicare billing practices, was “at the forefront of a national trend toward coding these relevant MCCs at a higher rate.” Id. at *4. Absent any insider information alleging otherwise, the court found that Integra offered only a “possible explanation” for the results of its statistical analysis (i.e., that Providence was directing its doctors to falsify claims) and ignored that the statistical analysis could also support a “plausible alternative (and legal) explanation.” Id. (emphasis in original). Thus, the court stated “[w]e need not accept the conclusion that the defendant engaged in unlawful conduct when its actions are in line with lawful ‘rational and competitive business strategy.’” Id. (citation omitted).
Although the Ninth Circuit’s decision should reduce the weight courts are willing to attribute to the findings of statistical analyses at the pleading stage FCA cases, the court expressly noted in a footnote that its decision was not “categorically preclud[ing]” the use of statistical data to meet the FRCP 8(a) and 9(b) pleading requirements. Id. at *4 n.5.
Similarly, in Estate of Helmly v. Bethany Hospice and Palliative Care of Coastal Georgia, LLC, the Eleventh Circuit upheld the dismissal with prejudice of a qui tam suit brought by two former employees against Bethany Hospice, reasoning that allegations based on numerical probability are mere inferences that do not suffice to plead fraud with particularity under Rule 9(b). No. 20-11624, 2021 WL 1609823, at *6 (11th Cir. Apr. 26, 2021).
In Helmly, the relators alleged that the defendant hospice violated the FCA by submitting false claims when it billed the government for services provided to patients obtained through a kickback scheme. Id. at *1. They argued that because a significant number of Medicare recipients were referred to the hospice, and because “all or nearly all” of the patients at the hospice received coverage from Medicare, it was mathematically plausible that the hospice had submitted to the government claims for patients obtained under kickback agreements. Id. at *4-6.
The Eleventh Circuit rejected this argument as the basis for an FCA claim, holding that relators failed to plead the submission of an actual false claim. Id. at *6. In order to meet Rule 9(b)’s particularity requirement, a complaint “must allege actual submission of a false claim” and must do so with “some indicia of reliability.” Id., at *5 (citing Carrel v. AIDS Healthcare Found., Inc., 898 F.3d 1267, 1275 (11th Cir. 2018)) (internal quotation marks omitted). The Helmly court held that “numerical probability is not an indicium of reliability” sufficient to “meet Rule 9(b)’s particularity requirement.” Id. at *6. “[R]elators cannot ‘rely on mathematical probability to conclude that [a defendant] surely must have submitted a false claim at some point.’” Id. (quoting Carrel, 898 F.3d at 1277) (second alteration in original).
These decisions demonstrate that the pleading stage of an FCA claim requires greater specificity than many relators have typically supplied. Regardless of what the alleged core FCA claim may entail, courts are likely to require plaintiffs to clearly connect the dots and provide more concrete evidence of falsity to survive a motion to dismiss.
D. NINTH CIRCUIT AFFIRMS THE “FRAUD-ON-THE-FDA” THEORY
This past spring, the Ninth Circuit reaffirmed that “fraud-on-the-FDA” theories may state a valid FCA claim sufficient to survive a motion to dismiss in certain circumstances. United States ex rel. Dan Abrams Co. LLC v. Medtronic Inc., 850 Fed. App’x 508 (9th Cir. 2021). In Medtronic, the relator alleged, among other claims, that the defendant fraudulently obtained FDA 510(k) clearance for several devices used in spinal fusion surgeries. Id. at 510. According to the relator, some of these devices could only be used for a contraindicated use, and could not be used as indicated in defendant’s 510(k) submissions at all (the “Contraindicated-only Devices”). Id. As such, the relator alleged that these devices were not properly approved or cleared by the FDA and thus would have been ineligible for reimbursement under Medicare but for the defendant’s alleged fraud. Id. The district court dismissed these fraud-on-the-FDA allegations for failure to state a claim because the allegations were offered “solely as a predicate for the claim that the [devices] were intended for off-label use” and “the federal government allows reimbursement for off-label and even contraindicated uses.” Id. at 511.
The Ninth Circuit affirmed most of the district court’s dismissal of relators’ claims, but reversed the district court’s holding as to the Contraindicated-only Devices, holding that the FCA may serve as a vehicle to bring a fraud-on-the-FDA claim here. Citing United States ex rel. Campie v. Gilead Sciences, Inc., 862 F.3d 890, 899 (9th Cir. 2017), the court concluded that for the Contraindicated-only Devices, the relator did not merely allege off-label use; rather, the relator alleged that the devices were not properly cleared for any use by the FDA. Because the Contraindicated-only Devices could “only be used for their contraindicated use,” and disclosures about that intended use are “precisely those that the FDA considers in granting Class II certification,” the court held that Medtronic’s alleged fraud went “to the very essence of the bargain” and therefore could proceed as a fraud-on-the-FDA claim. Medtronic, 850 Fed. App’x at 511. Although the Ninth Circuit recognized that other jurisdictions had previously “cautioned against allowing claims under the [FCA] to wade into the FDA’s regulatory regime[,]” citing Campie, 862 F.3d. at 905, Ninth Circuit precedent allowed a relator’s fraud-on-the-FDA theory to move forward. Id.
Relator’s other claims—such as the allegation that the defendant promoted off-label and contraindicated uses of certain devices—were dismissed because the devices included those that could be used for their stated intended use but were contraindicated for use elsewhere. Id. *3. The panel affirmed dismissal of the relator’s claim that defendant violated the AKS by entering into improper rebate agreements with hospitals and offering kickbacks to physicians for certain business development events. Id. at *511–12. The Ninth Circuit stated that the AKS does not include discounts offered to providers if they are properly disclosed and reflected in charges to the federal program. Moreover, the relator failed to explain how defendant’s rebate agreement violated the statute or to state sufficiently specific allegations related to physician kickbacks. Id.
E. FOURTH CIRCUIT AFFIRMS THE BROAD REACH OF THE AKS AS A BASIS FOR FCA LIABILITY
The Fourth Circuit’s ruling earlier this year in United States v. Mallory, 988 F.3d 730 (4th Cir. 2021), serves as a reminder of the risk of compensating independent contractors for marketing activities in light of HHS-OIG guidance on whether such compensation falls within an AKS safe harbor. In Mallory, a laboratory that provided blood testing for cardiovascular disease and diabetes contracted with a consulting company to market and sell the blood tests. The consulting company received a base payment and a percentage of revenue based on the number of blood tests ordered. Based on the evidence presented at trial, the jury found that the laboratory’s revenue-based commission payments to its sales agents constituted improper remuneration that was intended to induce the sales agents to sell as many laboratory tests as possible. See United States ex rel. Lutz v. BlueWave Healthcare Consultants, Inc., No. 9:11-CV-1593-RMG, 2018 WL 11282049, at *1 (D.S.C. May 23, 2018), aff’d sub nom. United States v. Mallory, 988 F.3d 730 (4th Cir. 2021).
Defendants argued on appeal that the government failed to prove that the defendants “knowingly and willfully” violated the AKS and that, accordingly, the defendants could not have “knowingly” violated the FCA. Mallory, 988 F.3d at 736. The Fourth Circuit found those arguments unconvincing given that, in the course of attempting to assert an advice-of-counsel defense, the defendants were unable to “identify any specific legal opinion” that could support a “good-faith belief that their conduct . . . did not violate the Anti-Kickback Statute.” Id. at 739. To the contrary, the Government offered evidence that several attorneys had expressed concerns to the defendants regarding possible AKS violations in the arrangements. Id. at 736–37.
The defendants also argued on appeal that commissions to independent contractor salespeople do not constitute kickbacks under the AKS. Although the court noted that the AKS does contain a safe harbor for bona fide employment relationships, it explained that HHS-OIG “has expressly recognized that this safe harbor does not cover independent contractors.” Id. at 738. The court discussed the history of the statutory safe harbor for commissions paid to salespeople who are “employee[s]” that have a “bona fide employment relationship” with their employer, 42 U.S.C. § 1320a-7b(b)(3)(B), and HHS’s reasoning that if employers “desire to pay [ ] salesperson[s] on the basis of the amount of business they generate,” they “should make these salespersons employees” to avoid “civil or criminal prosecution.” 54 Fed. Reg. 3088, 3093 (Jan. 23, 1989). Because the amount of compensation in Mallory varied with the volume of the referrals, the court found that it fit squarely outside the bounds of the salesperson commission safe harbor. Mallory, 988 F.3d at 738.
The Fourth Circuit affirmed the jury’s findings and assessment of actual damages totaling more than $16 million for violations of FCA. Id. at 742; Lutz, 2018 WL 11282049, at *2–3. The court also affirmed the district court’s judgment, which totaled more than $100 million after the district court trebled the actual damages and added civil monetary penalties as required by the FCA. Lutz, 2018 WL 11282049, at *8.
F. SUPREME COURT DECLINES TO REVIEW SEVERAL IMPORTANT ISSUES UNDER THE FCA
1. SUPREME COURT REJECTS OPPORTUNITY TO REVIEW A SEVENTH CIRCUIT DECISION UPHOLDING DOJ AUTHORITY TO DISMISS CASES OVER OBJECTION OF RELATORS
In the final week of June, the Supreme Court denied a petition to review a Seventh Circuit decision regarding the proper standard to evaluate a government motion to dismiss a relator’s claim. See Cimznhca, LLC v. United States, No. 20-1138, 2021 WL 2637991 (U.S. June 28, 2021). Cimznhca’s appeal argued that the Seventh Circuit improperly expanded its jurisdiction by treating the government’s motion to dismiss also as a motion to intervene for purposes of dismissal, even though the government never sought to intervene.
As explained in Gibson Dunn’s 2020 Year-End Update and discussed above, DOJ has more regularly invoked its dismissal authority under 31 U.S.C. § 3730(c)(2)(A) since the Granston Memo was issued. In evaluating DOJ’s requests to dismiss, courts historically have split based on whether they followed the Ninth Circuit’s Sequoia Orange test or the D.C. Circuit’s Swift test in deciding whether the government may dismiss a qui tam case. Under the Sequoia Orange approach, the government may dismiss a qui tam case if: (1) it identifies a valid government purpose; (2) a rational relation exists between the dismissal and the accomplishment of that purpose; and (3) dismissal is not fraudulent, arbitrary and capricious, or illegal. United States ex rel. Sequoia Orange Co. v. Baird-Neece Packing Corp., 151 F.3d 1139, 1145 (9th Cir. 1998). The Swift test, by contrast, affords the government an “unfettered” right to dismiss a case such that the decision is “unreviewable” except in instances of “fraud on the court.” Swift v. United States, 318 F.3d 250, 252-53 (D.C. Cir. 2003). Both standards generally favor the government’s discretion, albeit to different degrees, and DOJ regularly argues in its motions to dismiss that it has sufficient discretion to dismiss a case under either standard.
In Cimznhca, the Seventh Circuit called the choice between the Sequoia Orange and Swift standards “a false one, based on a misunderstanding of the government’s rights and obligations under the False Claims Act.” United States v. UCB, Inc., 970 F.3d 835, 839 (7th Cir. 2020). Although it recognized the value of a Sequoia Orange-type standard focused on the outer constitutional limits on the exercise of the government’s prosecutorial discretion, the court stated that it believes the limit lies closer to the more-deferential Swift standard.
When moving for dismissal in the district court, the government argued that the allegations “lack[ed] sufficient merit to justify the cost of investigation and prosecution and [were] otherwise . . . contrary to the public interest.” Id. at 840. In reversing the district court’s denial of the government’s motion, the Seventh Circuit viewed the government’s motion as a motion to intervene and dismiss and held that Federal Rule of Civil Procedure 41 (which governs voluntary dismissal by plaintiffs generally) supplied “the beginning and end of [the court’s] analysis.” Id. at 849. Turning to the Sequoia Orange and Swift standards, the court held that Sequoia Orange simply means that dismissal “may not violate the substantive component of the Due Process Clause,” id. at 851, which the court characterized as a “bare rationality standard” targeting “only the most egregious official conduct” that “shocks the conscience” or “offend[s] even hardened sensibilities,” id. at 852 (internal quotation marks omitted) (alteration in original). The court rejected the idea that the relatively formal nature of Section 3730(c)(2)(A) hearings “justif[ies] imposing on the government in each case the burden of satisfying Sequoia Orange’s ‘two-step test’ before the burden is put back on the relator to show unlawful executive conduct.” Id. at 853.
By declining to review the Cimznhca appeal, the Supreme Court left unresolved a growing circuit split over DOJ dismissals of whistleblower lawsuits. Accordingly, we may see other circuits apply either Sequoia Orange or Swift—or take the Seventh Circuit’s position in Cimznhca that the standard lies somewhere between the two and should primarily be informed by Federal Rule of Civil Procedure 41.
2. SUPREME COURT DECLINES TO RESOLVE DEBATE OVER “OBJECTIVELY FALSE”
In February, the United States Supreme Court also declined to resolve a prominent split between federal courts of appeal regarding the FCA’s falsity standard. In denying petitions for writs of certiorari in Care Alternatives v. United States, — S. Ct. —, 2021 WL 666386 (Feb. 22, 2021), and RollinsNelson LTC Corp. v. U.S. ex rel. Winters, — S. Ct. —, 2021 WL 666435 (Feb. 22, 2021), the Court left unresolved whether FCA liability must be predicated on a claim that is objectively false based on verifiable facts, or whether a post hoc expert opinion can suffice to establish falsity (at least at the pleading stage). As we have written about here, this objective falsity issue joins a host of other FCA-related questions as to which the federal courts have been unable to provide uniform answers.
IV. CONCLUSION
We will monitor these developments, along with other FCA legislative activity, settlements, and jurisprudence throughout the year and report back in our 2021 False Claims Act Year-End Update, which we will publish in January 2022.
____________________________
[1] See Press Release, U.S. Atty’s Office for the Western Dist. of WI, AutoGenomics, Inc. Agrees to Pay Over $2.5 Million for Allegedly Paying Kickbacks (Jan. 11, 2021), https://www.justice.gov/usao-wdwi/pr/autogenomics-inc-agrees-pay-over-25-million-allegedly-paying-kickbacks.
[2] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Florida Businesswoman Pleads Guilty to Criminal Health Care and Tax Fraud Charges and Agrees to $20.3 Million Civil False Claims Act Settlement (Feb. 4, 2021), https://www.justice.gov/opa/pr/florida-businesswoman-pleads-guilty-criminal-health-care-and-tax-fraud-charges-and-agrees-203.
[3] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, Lancaster County Pharmacy and Pharmacist Agree to Resolve Civil Allegations of Dispensing Controlled Substances Without a Prescription and Falsely Billing Medicare for $2.9 Million (Feb. 25, 2021), https://www.justice.gov/usao-edpa/pr/lancaster-county-pharmacy-and-pharmacist-agree-resolve-civil-allegations-dispensing.
[4] See Press Release, U.S. Atty’s Office for the Middle Dist. of NC, Bioventus Agrees to Pay More Than $3.6 Million to Resolve False Claims Act Violations (Feb. 25, 2021), https://www.justice.gov/usao-mdnc/pr/bioventus-agrees-pay-more-36-million-resolve-false-claims-act-violations.
[5] See Press Release, U.S. Atty’s Office for the Eastern Dist. of N.C., North Carolina Durable Medical Equipment Corporation Sentenced for $10 Million Healthcare Fraud Scheme, and the Company and Its Owner Agree to Pay Millions to Resolve Related Civil Claims (Mar. 2, 2021), https://www.justice.gov/usao-ednc/pr/north-carolina-durable-medical-equipment-corporation-sentenced-10-million-healthcare.
[6] See Press Release, U.S. Atty’s Office for the Western Dist. of VA, Allergy and Asthma Associates in Roanoke Pleads Guilty to Criminal Charge; Enters into Civil Resolution Over Health Care Fraud Allegations (Mar. 2, 2021), https://www.justice.gov/usao-wdva/pr/allergy-and-asthma-associates-roanoke-pleads-guilty-criminal-charge-enters-civil.
[7] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Ohio Treatment Facilities and Corporate Parent Agree to Pay $10.25 Million to Resolve False Claims Act Allegations of Kickbacks to Patients and Unnecessary Admissions (Mar. 5, 2021), https://www.justice.gov/opa/pr/ohio-treatment-facilities-and-corporate-parent-agree-pay-1025-million-resolve-false-claims.
[8] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Former Owners of Telemarketing Company Agree to Pay At Least $4 Million to Resolve False Claims Act Allegations (Mar. 16, 2021), https://www.justice.gov/opa/pr/former-owners-telemarketing-company-agree-pay-least-4-million-resolve-false-claims-act.
[9] See Press Release, U.S. Atty’s Office for the Eastern Dist. of MI, Cardiologist Dinesh Shah Pays $2 Million to Resolve False Claims Act Allegations Relating to Excessive Testing (Mar. 18, 2021), https://www.justice.gov/usao-edmi/pr/cardiologist-dinesh-shah-pays-2-million-resolve-false-claims-act-allegations-relating.
[10] See Press Release, U.S. Atty’s Office for the Western Dist. of NC, Owner of Defunct Urine Drug Testing Laboratory Agrees to Pay Over $2 Million to Resolve Allegations of Participation in Kickback Schemes (Mar. 26, 2021), https://www.justice.gov/usao-wdnc/pr/owner-defunct-urine-drug-testing-laboratory-agrees-pay-over-2-million-resolve; Press Release, U.S. Atty’s Office for the Western Dist. of NC, Court Enters $4.5 Million Judgment Against Owner of Defunct Urine Drug Testing Laboratory Resolving Allegations of Participation in Kickback Schemes (Mar. 30, 2021), https://www.justice.gov/usao-wdnc/pr/court-enters-45-million-judgment-against-owner-defunct-urine-drug-testing-laboratory.
[11] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, Bristol-Myers Squibb to Pay $75 Million to Resolve False Claims Act Allegations of Underpayment of Drug Rebates Owed Through Medicaid (Apr. 1, 2021), https://www.justice.gov/usao-edpa/pr/bristol-myers-squibb-pay-75-million-resolve-false-claims-act-allegations-underpayment.
[12] See Press Release, U.S. Atty’s Office for the Dist. of SC, South Carolina’s Largest Urgent Care Provider and its Management Company to Pay $22.5 Million to Settle False Claims Act Allegations (Apr. 8, 2021), https://www.justice.gov/usao-sc/pr/south-carolina-s-largest-urgent-care-provider-and-its-management-company-pay-225-million.
[13] See Press Release, U.S. Atty’s Office for the Dist. of MA, Massachusetts Eye and Ear Agrees to Pay $2.6 Million to Resolve False Claims Act Allegations (Apr. 20, 2021), https://www.justice.gov/usao-ma/pr/massachusetts-eye-and-ear-agrees-pay-26-million-resolve-false-claims-act-allegations.
[14] See Press Release, U.S. Atty’s Office for Middle Dist. of TN, Comprehensive Pain Specialists And Former Owners Agree To Pay $4.1 Million To Settle Fraud Allegations (Apr. 21, 2021), https://www.justice.gov/usao-mdtn/pr/comprehensive-pain-specialists-and-former-owners-agree-pay-41-million-settle-fraud.
[15] See Press Release, U.S. Atty’s Office for the Southern Dist. of FL, Miami-Based CareCloud Health, Inc. Agrees to Pay $3.8 Million to Resolve Allegations that it Paid Illegal Kickbacks (Apr. 30, 2021), https://www.justice.gov/usao-sdfl/pr/miami-based-carecloud-health-inc-agrees-pay-38-million-resolve-allegations-it-paid.
[16] See Press Release, U.S. Atty’s Office for the Dist. of SD, Neurosurgeon and Two Affiliated Companies Agree to Pay $4.4 Million to Settle Healthcare Fraud Allegations (May 3, 2021), https://www.justice.gov/usao-sd/pr/neurosurgeon-and-two-affiliated-companies-agree-pay-44-million-settle-healthcare-fraud; Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Neurosurgeon and Two Affiliated Companies Agree to Pay $4.4 Million to Settle Healthcare Fraud Allegations (May 3, 2021), https://www.justice.gov/opa/pr/neurosurgeon-and-two-affiliated-companies-agree-pay-44-million-settle-health-care-fraud.
[17] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, Pharmaceutical Manufacturer Agrees to Pay $12.6 Million to Resolve Allegations it Provided Kickbacks Through Donations to a Third-Party Charity (May 4, 2021), https://www.justice.gov/usao-edpa/pr/pharmaceutical-manufacturer-agrees-pay-126-million-resolve-allegations-it-provided; Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Incyte Corporation to Pay $12.6 Million to Resolve False Claims Act Allegations for Paying Kickbacks (May 4, 2021), https://www.justice.gov/opa/pr/incyte-corporation-pay-126-million-resolve-false-claims-act-allegations-paying-kickbacks.
[18] See Press Release, U.S. Atty’s Office for the Dist. of AZ, Neurosurgical Associates, LTD and Dignity Health, D/B/A St. Joseph’s Hospital, Paid $10 Million to Resolve False Claims Allegations (May 5, 2021), https://www.justice.gov/usao-az/pr/neurosurgical-associates-ltd-and-dignity-health-dba-st-josephs-hospital-paid-10-million.
[19] See Press Release, U.S. Atty’s Office for the Southern Dist. of FL, University of Miami to Pay $22 Million to Settle Claims Involving Medically Unnecessary Laboratory Tests and Fraudulent Billing Practices (May 10, 2021), https://www.justice.gov/usao-sdfl/pr/university-miami-pay-22-million-settle-claims-involving-medically-unnecessary; Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, University of Miami to Pay $22 Million to Settle Claims Involving Medically Unnecessary Laboratory Tests and Fraudulent Billing Practices (May 10, 2021), https://www.justice.gov/opa/pr/university-miami-pay-22-million-settle-claims-involving-medically-unnecessary-laboratory; Office of Inspector Gen. of Dep’t of Health and Hum. Servs., Corporate Integrity Agreement Between the Office of Inspector General of the Department of Health and Human Services and University of Miami (2021), https://oig.hhs.gov/fraud/cia/agreements/University_of_Miami_05072021.pdf.
[20] See Press Release, U.S. Atty’s Office for the Northern Dist. of GA, AlixaRx LLC Agrees to Pay $2.75 Million to Resolve Allegations that it Improperly Dispensed Controlled Substances at Long-Term Care Facilities (May 11, 2021), https://www.justice.gov/usao-ndga/pr/alixarx-llc-agrees-pay-275-million-resolve-allegations-it-improperly-dispensed.
[21] See Press Release, U.S. Atty’s Office for the Northern Dist. of TX, Dentists to Pay $3.1 Million to Resolve Allegations They Submitted False Claims for Services Not Provided to Underprivileged Children (May 14, 2021), https://www.justice.gov/usao-ndtx/pr/dentists-pay-31-million-resolve-allegations-they-submitted-false-claims-services-not.
[22] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, French Medical Device Manufacturer to Pay $2 Million to Resolve Alleged Kickbacks to Physicians and Related Medicare Open Payments Program Violations (May 19, 2021), https://www.justice.gov/usao-edpa/pr/french-medical-device-manufacturer-pay-2-million-resolve-alleged-kickbacks-physicians.
[23] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, Atlanta-Based National Chain of Skilled Nursing Facilities to Pay $11.2 Million to Resolve Allegations of Providing Substandard Care, Medically Unnecessary Therapy Services (May 21, 2021), https://www.justice.gov/usao-edpa/pr/atlanta-based-national-chain-skilled-nursing-facilities-pay-112-million-resolve.
[24] See Press Release, U.S. Atty’s Office for the Western Dist. of NY, Upper Allegheny Health System To Pay $2.7 Million To Settle False Claims Act Allegations (May 25, 2021), https://www.justice.gov/usao-wdny/pr/upper-allegheny-health-system-pay-27-million-settle-false-claims-act-allegations.
[25] See Press Release, U.S. Atty’s Office for the Southern Dist. of TX, Wrongful Billing Results in $2.6M Settlement and 10-Year Exclusion from Federal Health Care Programs (June 8, 2021), https://www.justice.gov/usao-sdtx/pr/wrongful-billing-results-26m-settlement-and-10-year-exclusion-federal-health-care.
[26] See Press Release, U.S. Atty’s Office for the Middle Dist. of FL, Surgical Care Affiliates And Orlando Surgery Center Agree To Pay $3.4 Million To Settle False Claims Act Liability (June 28, 2021), https://www.justice.gov/usao-mdfl/pr/surgical-care-affiliates-and-orlando-surgery-center-agree-pay-34-million-settle-false.
[27] See Press Release, U.S. Atty’s Office for the Dist. of CT, Connecticut Electrical Contractor Agrees to Pay $3.2 Million to Resolve Criminal and Civil Investigation (Jan. 8, 2021), https://www.justice.gov/usao-ct/pr/connecticut-electrical-contractor-agrees-pay-32-million-resolve-criminal-and-civil.
[28] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Insitu Inc. to Pay $25 Million to Settle False Claims Act Case Alleging Knowing Overcharges on Unmanned Aerial Vehicle Contracts (Jan. 12, 2021), https://www.justice.gov/opa/pr/insitu-inc-pay-25-million-settle-false-claims-act-case-alleging-knowing-overcharges-unmanned.
[29] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Concrete Contractor Agrees to Settle False Claims Act Allegations for $3.9 Million (Feb. 17, 2021), https://www.justice.gov/opa/pr/concrete-contractor-agrees-settle-false-claims-act-allegations-39-million.
[30] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Federal Contractor Agrees to Pay More Than $6 Million to Settle Overbilling Allegations (Feb. 19, 2021), https://www.justice.gov/opa/pr/federal-contractor-agrees-pay-more-6-million-settle-overbilling-allegations.
[31] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, United Airlines to Pay $49 Million to Resolve Criminal Fraud Charges and Civil Claims (Feb. 26, 2021), https://www.justice.gov/opa/pr/united-airlines-pay-49-million-resolve-criminal-fraud-charges-and-civil-claims.
[32] See Press Release, U.S. Atty’s Office for the Eastern Dist. of PA, SAP Public Services, Inc. to Pay $2.2 Million to Settle False Claims Act Allegations (Mar. 1, 2021), https://www.justice.gov/usao-edpa/pr/sap-public-services-inc-pay-22-million-settle-false-claims-act-allegations.
[33] See Press Release, U.S. Atty’s Office for D.C., The International Rescue Committee (“IRC”) Agrees to Pay $6.9 Million to Settle Allegations That It Performed Procurement Fraud by Engaging in Collusive Behavior and Misconduct on Programs Funded by the U.S. Agency for International Development (Mar. 19, 2021), https://www.justice.gov/usao-dc/pr/international-rescue-committee-irc-agrees-pay-69-million-settle-allegations-it-performed.
[34] See Press Release, U.S. Atty’s Office for Southern Dist. of CA, Tungsten Heavy Powder of San Diego Agrees to Pay $5.6 Million to Settle False Claims Act Allegations (Apr. 29, 2021), https://www.justice.gov/usao-sdca/pr/tungsten-heavy-powder-san-diego-agrees-pay-56-million-settle-false-claims-act.
[35] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Navistar Defense Agrees to Pay $50 Million to Resolve False Claims Act Allegations Involving Submission of Fraudulent Sales Histories (May 27, 2021), https://www.justice.gov/opa/pr/navistar-defense-agrees-pay-50-million-resolve-false-claims-act-allegations-involving.
[36] See Press Release, U.S. Atty’s Office for Eastern WA, CH2M Hill Plateau Remediation Company Agrees to Pay More than $3 Million to Settle Hanford Subcontract Small Business Fraud Allegations (June 3, 2021), https://www.justice.gov/usao-edwa/pr/ch2m-hill-plateau-remediation-company-agrees-pay-more-3-million-settle-hanford.
[37] See Press Release, U.S. Atty’s Office for NJ, Avis Budget Group to Pay $10.1 Million to Settle False Claims Act Allegations for Overcharging United States on Rental Vehicles (June 10, 2021), https://www.justice.gov/usao-nj/pr/avis-budget-group-pay-101-million-settle-false-claims-act-allegations-overcharging-united.
[38] See Press Release, U.S. Atty’s Office for the Eastern Dist. of VA, Level 3 Communications, LLC Agrees to Pay Over $12.7 Million to Settle Civil False Claims Act Allegations (June 25, 2021), https://www.justice.gov/usao-edva/pr/level-3-communications-llc-agrees-pay-over-127-million-settle-civil-false-claims-act.
[39] See Press Release, U.S. Atty’s Office for the Eastern Dist. of VA, Armed Forces Services Corporation Pays $4.3 Million to Resolve Anti-Kickback Act and False Claims Act Allegations (June 30, 2021), https://www.justice.gov/usao-edva/pr/armed-forces-services-corporation-pays-43-million-resolve-anti-kickback-act-and-false.
[40] Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Acting Assistant Attorney General Brian M. Boynton Delivers Remarks at the Federal Bar Association Qui Tam Conference (Feb. 17, 2021), https://www.justice.gov/opa/speech/acting-assistant-attorney-general-brian-m-boynton-delivers-remarks-federal-bar [hereinafter, “Boynton Speech”].
[43] See Press Release, U.S. Atty’s Office for the Eastern Dist. of CA., Eastern District of California Obtains Nation’s First Civil Settlement for Fraud on Cares Act Paycheck Protection Program (Jan. 12, 2021), https://www.justice.gov/usao-edca/pr/eastern-district-california-obtains-nation-s-first-civil-settlement-fraud-cares-act.
[44] Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Justice Department Takes Action Against COVID-19 Fraud: Historic level of enforcement action during national health emergency continues (Mar. 26, 2021), https://www.justice.gov/opa/pr/justice-department-takes-action-against-covid-19-fraud.
[45] Boynton Speech, supra note 41.
[47] U.S. Dep’t of Justice, Memorandum from Michael D. Granston, Director, Commercial Litigation Branch, Fraud Section (Jan. 10, 2018), https://drive.google.com/file/d/1PjNaQyopCs_KDWy8RL0QPAEIPTnv31ph/view.
[50] Ltr. from Sen. Chuck Grassley to Hon. Merrick B. Garland (Feb. 24, 2021), https://g7x5y3i9.rocketcdn.me/wp-content/uploads/2021/03/2021-02-24-CEG-to-DOJAG-Nominee-Garland-regarding-FCA.pdf [hereinafter, “Grassley Letter”].
[53] Department of Justice, Office of the Associate Attorney General Rachel Brand, Memorandum for Heads of Civil Litigating Components and United States Attorneys: Limiting Use of Agency Guidance Documents In Affirmative Civil Enforcement Cases (Jan. 25, 2018), available at https://www.justice.gov/file/1028756/download.
[57] Department of Justice, Justice Manual § 1-20.000, available at https://www.justice.gov/jm/1-20000-limitation-use-guidance-documents-litigation.
[58] Executive Order 13992, 86 Fed. Reg. 7049 (Jan. 20, 2021) (“Revocation of Certain Executive Orders Concerning Federal Regulation”).
[59] Executive Order 13981, 84 Fed. Reg. 55235 (Oct. 9, 2019) (“Promoting the Rule of Law Through Improved Agency Guidance Documents”).
[61] HHS-OIG, State False Claims Act Reviews, https://oig.hhs.gov/fraud/state-false-claims-act-reviews/.
[64] Id.; see also Ltr. from Christi A. Grimm, Principal Deputy Inspector General, to Hon. Keith Ellison, Attorney General of Minnesota (May 27, 2021), https://oig.hhs.gov/documents/false-claims-act/369/Minnesota_False_Claims_Act_Letter_05272021.pdf.
[65] State False Claims Act Reviews, supra note 62.
[66] See Montana S.B. No. 345, https://legiscan.com/MT/bill/SB345/2021.
[68] Arkansas H.B. 1623, https://legiscan.com/AR/text/HB1623/2021.
[69] California Assembly Bill No. 310, https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=202120220AB310.
The following Gibson Dunn lawyers assisted in the preparation of this alert: Jonathan Phillips, Winston Chan, Nicola Hanna, John Partridge, James Zelenay, Sean Twomey, Reid Rector, Allison Chapin, Maya Nuland, Michael Dziuban, and Eva Michaels.
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues. Please feel free to contact the Gibson Dunn lawyer with whom you usually work, the authors, or any of the following members of the firm’s False Claims Act/Qui Tam Defense Group:
False Claims Act/Qui Tam Defense Group Leaders:
Winston Y. Chan – San Francisco (+1 415-393-8362, wchan@gibsondunn.com)
Jonathan M. Phillips – Washington, D.C. (+1 202-887-3546, jphillips@gibsondunn.com)
Please also feel free to contact any of the following practice members:
Washington, D.C.
Jonathan M. Phillips (+1 202-887-3546, jphillips@gibsondunn.com),
F. Joseph Warin (+1 202-887-3609, fwarin@gibsondunn.com)
Joseph D. West (+1 202-955-8658, jwest@gibsondunn.com)
Robert K. Hur (+1 202-887-3674, rhur@gibsondunn.com)
Geoffrey M. Sigler (+1 202-887-3752, gsigler@gibsondunn.com)
New York
Reed Brodsky (+1 212-351-5334, rbrodsky@gibsondunn.com)
Mylan Denerstein (+1 212-351-3850, mdenerstein@gibsondunn.com)
Alexander H. Southwell (+1 212-351-3981, asouthwell@gibsondunn.com)
Casey Kyung-Se Lee (+1 212-351-2419, clee@gibsondunn.com)
Denver
Robert C. Blume (+1 303-298-5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303-298-5784, mloseman@gibsondunn.com)
John D.W. Partridge (+1 303-298-5931, jpartridge@gibsondunn.com)
Ryan T. Bergsieker (+1 303-298-5774, rbergsieker@gibsondunn.com)
Dallas
Robert C. Walters (+1 214-698-3114, rwalters@gibsondunn.com)
Andrew LeGrand (+1 214-698-3405, alegrand@gibsondunn.com)
Los Angeles
Nicola T. Hanna (+1 213-229-7269, nhanna@gibsondunn.com)
Timothy J. Hatch (+1 213-229-7368, thatch@gibsondunn.com)
Deborah L. Stein (+1 213-229-7164, dstein@gibsondunn.com)
James L. Zelenay Jr. (+1 213-229-7449, jzelenay@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650-849-5395, bwagner@gibsondunn.com)
San Francisco
Winston Y. Chan (+1 415-393-8362, wchan@gibsondunn.com)
Charles J. Stevens (+1 415-393-8391, cstevens@gibsondunn.com)
© 2021 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice.
Looking back on the incredible year that was 2020, some observers of the False Claims Act (“FCA”) enforcement space may note that the year’s FCA recoveries were the lowest they have been in twelve years, but the most important takeaway for those who deal in government funds is this: the government opened the most new FCA investigations ever in 2020. Despite the global pandemic, closed courts, and the realities of remote work (including remote investigations and litigation), the government and qui tam relators still opened 922 new FCA cases last year. This is the largest single-year total ever by a substantial margin and brings the total number of new FCA cases opened in the last 5 years to more than 4,100.
If the government’s enforcement activity around past economic crises and resulting government stimulus programs is any indication, the stage is set for FCA cases to surge further still in the next few years. Last year, the government enacted legislative stimulus packages totaling nearly $4 trillion in COVID-relief funds, and anytime the government spends money, FCA cases follow. A huge portion of that spending, moreover, has been in health care and health care-adjacent fields, areas which have accounted for more than 80% of all FCA recoveries over the last four years. Further, the Department of Justice (“DOJ”) swiftly prioritized rooting out COVID-related fraud in 2020—a focus that we expect to continue and likely intensify under the Biden administration. As the incoming administration’s enforcement priorities solidify, we also will monitor any efforts to change course from steps previously taken by the Trump administration toward reining in FCA enforcement through various policy changes, such as the Brand Memorandum’s prohibition of DOJ enforcement actions predicated on violations of non-binding agency guidance.[1]
Meanwhile, 2020 saw no major legislative developments relating to the FCA at the federal level. But states continue to enact or amend false claims statutes that enable states to receive a higher percentage share of recoveries and expand potential liability. On the judicial front, courts issued a number of significant decisions in 2020, including important decisions exploring the FCA’s materiality and scienter requirements, and several decisions regarding DOJ’s discretion to dismiss qui tam cases where the government has not intervened.
As always, Gibson Dunn’s recent publications on the FCA may be found on our website, including industry-specific articles, webcasts, presentations, and practical guidance to help companies avoid or limit liability under the FCA. And, of course, we would be happy to discuss these developments—and their implications for your business—with you.
I. FCA ENFORCEMENT ACTIVITY
A. New FCA Activity
The government and qui tam relators filed more FCA cases in 2020 (922) than in any other year since Congress enacted the FCA during the Civil War.[2] Although that figure is staggering in and of itself, equally surprising is who drove the increase in cases.[3]
During the last five years, there has been an average of approximately 800 new FCA cases a year, with qui tam relators filing approximately 660 cases on average and the government filing approximately 135 cases on average. But in 2020, the federal government was the impetus behind the increase to more than 900 new cases. These non-qui tam cases may arise from a variety of sources, including referrals from government agencies based on their program oversight activities or from mining government spending data for leads. With 250 cases last year, federal enforcement attorneys filed 120 more cases than in an average year, a mark last seen in 1994 when the modern qui tam provisions were still relatively new. As discussed below in the following section, cases where the government is involved—either because the government brought the case, or later intervened—typically account for 90% of all FCA cases with a recovery. The fact that the government brought so many new cases in 2020 suggests that recoveries in years to come will be robust.
Some of the government’s new cases stem from COVID relief efforts and a desire to police fraud in the government’s massive spending programs during the last year. But it does not appear that COVID-related cases account for the entirety of the nearly 100% increase in cases by the government. As more details are released about those cases, we will be watching carefully to identify where the government’s actions are focused.
Number of FCA New Matters, Including Qui Tam Actions
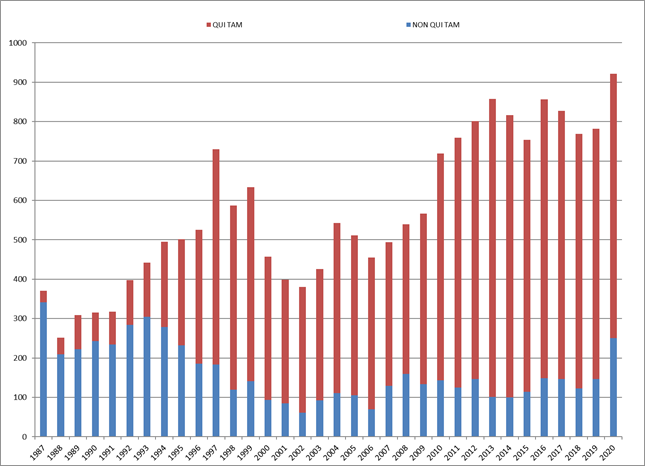
Source: DOJ “Fraud Statistics – Overview” (Jan. 14, 2021)
B. Total Recovery Amounts: 2020 Recoveries Exceed $2 Billion
The federal government also recovered more than $2.2 billion during fiscal year 2020, which ended September 30, 2020. Of this amount, more than 90% was recovered in intervened cases, underscoring that companies face more significant exposure in cases in which the government initiated the case or intervened.
The total of $2.2 billion is down from recent years, as shown in the chart below. Given the continued high number of new investigations being opened, this is likely a reflection of disruptions caused by the COVID-19 pandemic. Although COVID never resulted in a total work stoppage, investigations were delayed as were court proceedings in the middle of 2020. As noted, however, the overall pace of FCA litigation has not slowed whatsoever, and the pipeline of new cases is as full as ever. Significant settlements entered into after the close of fiscal year 2020, such as the $2.8 billion settlement entered into with an opioid manufacturer discussed below, are likewise poised to boost next fiscal year’s figures drastically.
Settlements or Judgments in Cases Where the Government Declined Intervention as a Percentage of Total FCA Recoveries
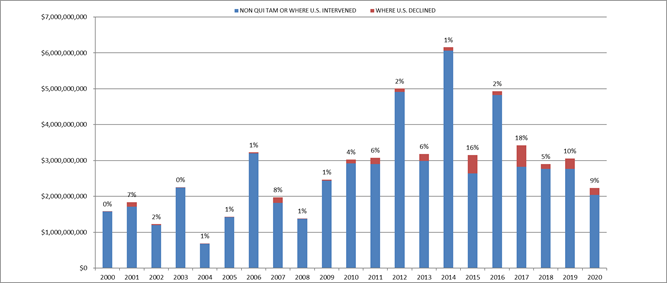
Source: DOJ “Fraud Statistics – Overview” (Jan. 14, 2021)
C. Industry Breakdown
While filings are up and recoveries are (perhaps temporarily) down, the industry breakdown of recoveries remains largely unchanged. As Assistant Attorney General Michael D. Granston recently remarked at the ABA Civil False Claims Act and Qui Tam Enforcement Institute, “[o]f the $11.4 billion recovered over the last four years, approximately 80 percent, or $9 billion, was recovered in health care fraud matters,” while “procurement fraud and mortgage fraud” marked the next two largest categories.[4]
2020 was no exception: Health care cases comprised 83% of total recoveries, Department of Defense procurement issues made up 3%, and the remaining 14% was split among other areas.[5]
FCA Recoveries by Industry
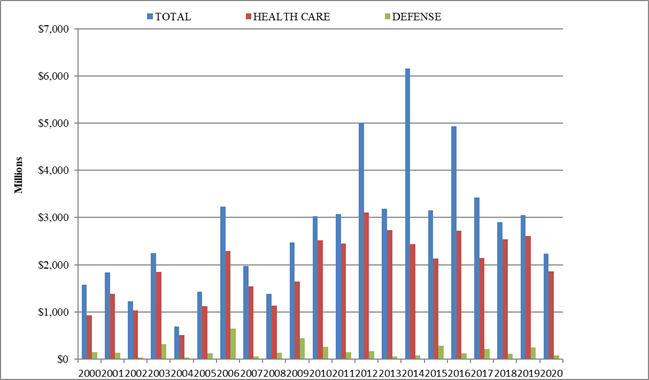
Source: DOJ “Fraud Statistics – Overview” (Jan. 14, 2021)
II. NOTEWORTHY DOJ ENFORCEMENT ACTIVITY DURING THE SECOND HALF OF 2020
We summarize below some of the notable FCA settlements announced since July 2020 (we covered notable settlements and judgments from the first half of 2020 in our 2020 Mid-Year False Claims Act Update). These summaries provide insight into the theories of liability and industries that have been a focus of government (and relator) enforcement efforts during the last year.
A. Health Care and Life Science Industries
- On July 1, a molecular diagnostics testing company agreed to pay $8.25 million to settle allegations that it violated the FCA by conspiring with hospitals to artificially delay orders for the company’s genetic test. The company allegedly sought to circumvent Medicare’s 14-Day Rule, which prohibited laboratories from separately billing for certain tests ordered within 14 days of a patient’s discharge from an inpatient or outpatient hospital setting. The government previously alleged in a separate settlement in 2017 that a Kentucky hospital also participated in the scheme in which the company separately billed Medicare instead of the hospital for the tests. A former employee initially filed the qui tam lawsuit, and the whistleblower’s share was not disclosed at the time of the settlement announcement.[6]
- On July 1, a pharmaceutical company agreed to pay $678 million to resolve claims that it violated the FCA. As part of the settlement, the company agreed to pay $51.25 million to resolve allegations that it improperly used three foundations as conduits to pay copayments of Medicare patients taking its drugs in a manner that resulted in disproportionate assistance for those patients. The company also agreed to pay $591.44 million to resolve allegations that it paid kickbacks through speaker programs and related events. As purported inducement to prescribe its products, the company’s managers allegedly instructed sales representatives to select high-volume prescribers as paid speakers. The company further agreed to forfeit $38.4 million, to pay approximately $48 million to resolve state claims, and to abide by strict limitations on future speaker programs and other events under a five-year Corporate Integrity Agreement.[7]
- On July 8, a hospice care company agreed to pay $3.2 million to settle claims that it violated the FCA by knowingly submitting false claims to Medicare, Medicaid, and TRICARE for hospice care provided to purportedly non-terminally-ill beneficiaries who did not qualify for those services. The settlement also resolves allegations that the company submitted false claims for a medically unnecessary level of hospice care. The company agreed to enter into a Corporate Integrity Agreement as part of the settlement, and the whistleblower, a former employee, will receive 19% of the recovery.[8]
- On July 8, an Oklahoma City-based specialty hospital, its part-owner and management company, a physician group, and two physicians agreed to pay $72.3 million to resolve allegations that they violated the FCA and the Oklahoma Medicaid False Claims Act. The government alleged that improper relationships between the specialty hospital and physician group resulted in the submission of false claims to Medicare, Medicaid and TRICARE, and that the specialty hospital and its management company provided improper remuneration to the physician group and certain physicians in exchange for referrals. The settlement also resolves claims related to the management company’s purportedly preferential offering of investment opportunities to physicians at four surgery facilities in Texas. The specialty hospital agreed to pay $60.86 million to the United States, $5 million to Oklahoma, and $206,000 to Texas. The physician group and two of its physicians, agreed to pay $5.7 million to the United States and $495,619 to Oklahoma. The specialty hospital and the physician group also agreed to enter five-year Corporate Integrity Agreements. The whistleblower’s share had not yet been determined at the time of the settlement announcement.[9]
- On July 10, a hospital management company, its subsidiary, and one of its facilities agreed to pay a total of $122 million to resolve alleged violations of the FCA. The management company and its subsidiary agreed to pay a total of $117 million, split between the United States and participating states, to resolve allegations that they submitted false claims to Medicare, Medicaid, TRICARE, Department of Veterans Affairs, and Federal Employee Health Benefit programs for billing for medically unnecessary inpatient behavior health services and failing to provide appropriate and adequate services to patients. The company expressly denied the allegations. In a separate settlement, the facility agreed to pay the United States and the State of Georgia $5 million to resolve allegations that it provided free or discounted transportation services to induce Medicare and Medicaid beneficiaries to seek treatment at certain of the facility’s programs. The management company, on behalf of its inpatient acute and residential behavioral health facilities, also agreed to enter into a five-year corporate integrity agreement. The settlement with the management company resolves 18 qui tam lawsuits, and the whistleblowers will receive a total of $15.86 million of the federal recovery. The settlement with the facility stemmed from a separate qui tam lawsuit, and the whistleblower will receive $861,853 from the recovery.[10]
- On July 13, a management corporation and 27 affiliated skilled nursing facilities agreed to pay $16.7 million to settle allegations that they violated the FCA by submitting false claims to Medicare for unnecessary or unreasonable rehabilitation therapy services. The facilities allegedly pressured therapists to increase the amount of patient therapy to meet pre-planned Medicare revenue targets, purportedly set without regard to patients’ needs and at an amount achievable only by billing high percentages of patients at the highest Medicare reimbursement level. The company entered into a five-year Corporate Integrity Agreement, and the whistleblowers collectively will receive approximately $3 million of the recovery.[11]
- On July 20, a health care company agreed to pay $11.94 million to resolve allegations that the company violated the FCA and Anti-Kickback Statute (“AKS”) by paying kickbacks to two companies in exchange for referrals of urine drug tests paid for by federal healthcare programs. The company agreed to fully cooperate and enter a five-year Corporate Integrity Agreement, under which the company must routinely report to the Office of Inspector General for the United States Department of Health and Human Services (“HHS-OIG”) and retain an Independent Review Organization to monitor its arrangements with other individuals and entities. One of the companies receiving the kickbacks and three of its executives also were indicted for conspiracy to pay and solicit kickbacks. The trial is set to take place in 2021.[12]
- On July 23, a biotech testing company agreed to pay $49 million to resolve allegations that the company fraudulently overbilled Medicaid and the Department of Veterans Affairs by miscoding its prenatal tests and that it provided illegal kickbacks to physicians in the form of excessive “draw fees,” meals and happy hours, and improperly reduced or waived patient coinsurance and deductible payments to induce orders for the company’s tests. The company agreed to pay $19.45 million to the United States and $13.15 million to various states to resolve the kickback and fraudulent billing claims and agreed to enter a five-year Corporate Integrity Agreement. The allegations stemmed from a qui tam lawsuit; the whistleblower’s share in the recovery had not been announced at the time of settlement. In a separate settlement, the company agreed to pay $16.4 million to resolve similar fraudulent billing claims related to TRICARE and the Federal Employees Health Benefits Program with the U.S. Attorney’s Office for the Southern District of California, and the company entered into a Non-Prosecution Agreement with that office.[13]
- On July 24, a pharmaceutical company’s two parent companies agreed to pay $300 million to resolve allegations that they caused the submission of false claims to government health care programs in violation of the FCA. The government alleged that the companies improperly promoted the sale and use of an opioid-addiction-treatment drug to physicians for indications that were not medically accepted, among other allegations. The government also alleged that the companies promoted the drug to physicians and state Medicaid agencies using false and misleading claims regarding the diversion, abuse, and safety risks of the drug, and that they took steps to improperly control the pricing of the drug by seeking to delay the entry of generic competitors, including through a petition to the U.S. Food and Drug Administration (“FDA”) claiming safety issues with the drug’s tablet version. Approximately $209.3 million of the civil settlement will go to the federal government and $90.7 million will go to states opting in to the agreement. The civil settlement stemmed from six qui tam lawsuits, and the whistleblowers’ share in the recovery had not been announced at the time of settlement. Separately, the pharmaceutical company agreed to pay another $289 in a criminal fine, forfeiture, and restitution in connection with pleading guilty to a one-count felony charge for making false statements relating to health care matters in connection with marketing and promoting the safety of its products, and the former CEO of its parent pleaded guilty to a one-count misdemeanor information related to the company’s alleged false and misleading representations to the Massachusetts Medicaid program. The pharmaceutical company also entered into a five-year Corporate Integrity Agreement that includes numerous accountability and auditing provisions as part of the resolution, and the company separately agreed to pay $10 million to the FTC to resolve unfair competition claims. The settlements come on the heels of a $1.4 billion resolution with the pharmaceutical company’s former parent, announced in 2019, which also related to the marketing of the company’s opioid drug.[14]
- On July 28, a pharmaceutical company agreed to pay $3.5 million to resolve claims that it violated the FCA by paying kickbacks to physicians through sham research grants as inducement to prescribe the company’s newly-launched analgesic drug. Among other allegations, the pharmaceutical company purportedly required placement of its drug on the formulary of the physicians’ institution before agreeing to award research grants and subsequently expressed little interest in the physicians’ proposed research. The whistleblower, a pharmacist, will receive approximately $520,000 of the federal recovery and approximately $118,000 of the state recovery.[15]
- On August 19, a nonprofit hospice provider agreed to pay $5.2 million to settle allegations that it improperly billed Medicare and Medicaid for services provided to hospice patients at unnecessarily heightened levels of care for which the patients did not qualify. The provider agreed to pay $4.85 million to the United States and agreed to pay $375,000 to New York. The allegations stem from a qui tam lawsuit, and the whistleblower’s share in the recovery was not disclosed at the time of the settlement announcement.[16]
- On August 24, a Massachusetts-based pharmaceuticals company agreed to pay $20.75 million to settle claims that it knowingly promoted a drug administration process that contradicted the FDA-approved instructions and was unsupported by sufficient clinical evidence, thereby causing physicians to submit false claims to Medicare and the Federal Employee Health Benefit Program. The company allegedly encouraged physicians to use a less effective drug administration process through paid speaker programs and physician peer-to-peer discussions, promotion by the company’s sales personnel, and dissemination of incomplete or misleading responses to questions asked by physicians, among other means. The company also allegedly failed to inform physicians that the administration process resulted in significantly lower clearance rates for the condition and, at times, falsely stated that the clearance rates were the same. The company and its parent company agreed to enter a Corporate Integrity Agreement, and the whistleblower, a former sales representative, will receive approximately $3.5 million of the recovery.[17]
- On September 9, a West Virginia-based acute care hospital agreed to pay $50 million to resolve allegations that it paid illegal kickbacks under the FCA to referring physicians. The government alleged that, over thirteen years, the hospital improperly paid the physicians based on the volume or value of their referrals, or otherwise paid them above-fair-market-value rates. The whistleblower will receive $10 million of the recovery.[18]
- Also on September 9, two companies that operate eleven radiology facilities in California agreed to pay $5 million to resolve allegations that they knowingly submitted claims for improperly supervised CT scans and MRIs in violation of the FCA. The companies also agreed to enter into a three-year Integrity Agreement with HHS-OIG. The whistleblower will receive approximately $925,000 of the recovery.[19]
- On September 11, a research institute agreed to pay $10 million to settle allegations that for a period of eight years it improperly charged research grants funded by the National Institutes of Health for activities unrelated to the grants, such as faculty time spent writing new grant applications, teaching, administrative activities, and committee tasks. The whistleblower will receive $1.75 million of the recovery.[20]
- On September 22, a biotechnology company that provides molecular and diagnostic tests agreed to pay $11.5 million to resolve claims that it knowingly billed government healthcare programs for inpatient testing for which the hospitals should have paid, and that it paid a percentage of the cost of electronic medical records transition software for sixty-nine physicians’ offices that the company calculated would generate revenue for the company equal to three times its payment.[21] The company made several admissions related to the purported conduct as part of the settlement.
- On September 23, a pharmaceutical company joined the growing list of companies to face FCA liability for allegedly setting up a fund within a charitable foundation to pay the co-pays of Medicare patients using the company’s pulmonary arterial hypertension drug. The government alleged that the company used spend data from the foundation to assess the amount patients were paying for its drug, then made charitable donations to the foundation sufficient to cover only those payments while simultaneously referring patients to the foundation. The company entered into a $97 million settlement to resolve the matter, without admitting any wrongdoing.[22]
- On September 28, a Texas-based hospital and co-defendants agreed to pay more than $15.3 million to resolve allegations that they overstated support and understated risks of construction of the hospital in order to obtain a federal mortgage loan, including by delaying refunds for cancelled investments, resulting in a loss for the U.S. Department of Housing and Urban Development, which had purchased the mortgage note.[23]
- On October 14, a medical device maker settled allegations that for a period of six years it paid kickbacks in the form of free advertising and practice support to physicians and hospitals in exchange for referrals of its embolization devices. DOJ alleged that the device maker ignored numerous warnings that its conduct may violate the AKS, including from its own Chief Compliance Officer. To settle the allegations, the device maker agreed to pay $18 million and enter into a five-year Corporate Integrity Agreement with HHS-OIG, pursuant to which it must hire a compliance expert and undergo review by an independent review organization. The whistleblower will receive $2.65 million.[24]
- On October 21, an opioid manufacturer agreed to pay $2.8 billion to resolve allegations that it promoted opioids for uses that were unsafe and medically unnecessary and engaged in kickback schemes to induce physicians to prescribe its drugs. With respect to the AKS, DOJ alleged that the manufacturer paid physicians to prescribe its opioids under the guise of payments for educational talks and consultant agreements; paid an electronic health records company to facilitate referrals, recommendations, and orders of its opioids; and contracted with specialty pharmacies to fill prescriptions other pharmacies had rejected. The manufacturer’s settlement is part of a broader global resolution, pursuant to which the manufacturer agreed to pay $8.3 billion to settle the FCA allegations and related criminal charges.[25]
- On October 29, a medical device maker agreed to pay $8.1 million to resolve allegations that, in order to induce a neurosurgeon to use the device maker’s implantable pumps, it paid for meals and drinks at more than one hundred social events hosted at a restaurant owned by the neurosurgeon and his wife and attended by the neurosurgeon’s acquaintances, colleagues, and existing and potential referral sources.[26]
- On November 16, a Medicare Advantage provider agreed to pay over $6.3 million to settle allegations that it violated the FCA by knowingly submitting invalid diagnoses to Medicare that were not supported by Medicare Advantage beneficiaries’ medical records. These submissions allegedly resulted in inflated payments from Medicare. The allegations stem from a qui tam lawsuit brought by a former employee. The whistleblower will receive approximately $1.5 million of the recovery.[27]
- On November 19, the former owners of a drug and device subsidiary agreed to pay $10 million to resolve allegations that the subsidiary violated the FCA by promoting two systems for unapproved uses for pediatric patients between 2006 and 2012. A private equity company that also formerly owned the subsidiary agreed to pay an additional $1.5 million to settle allegations that the subsidiary continued the allegedly improper practices after that owner acquired the company in 2012. The allegations stem from a qui tam lawsuit, and the whistleblowers’ share of the settlement was not announced at the time of the settlement announcement.[28]
- On November 20, a Florida-based home health agency and two former executives agreed to pay $5.8 million in total to settle allegations that the home health agency provided improper financial inducements to referring physicians in violation of the FCA. The home health agency paid just over $3.85 million and each executive paid $647,000. The government alleged that the home health agency violated the AKS and the Stark Law by entering into fake medical director agreements as a way of providing remuneration for referrals. The government also alleged that the home health agency violated the Stark Law by providing bonuses to employees based on referrals made by their physician spouses. The home health agency also agreed to pay an additional $675,000 to settle separate allegations that its employees pressured clinical personnel to increase the number of home visits to Medicare patients to avoid a Medicare adjustment that would have decreased the home health agency’s Medicare reimbursement. The government alleged that these services were medically unnecessary. The allegations stem from two qui tam lawsuits. The relators in one lawsuit received approximately $145,000 of the proceeds related to the Medicare adjustment allegations, and the relator’s share in the other lawsuit had yet to be determined at the time of the settlement announcement.[29]
- On December 17, a Massachusetts-based pharmaceutical company agreed to pay $22 million to resolve allegations that it violated the FCA by illegally using two foundations as a conduit to pay copays for Medicare patients to induce the patients to fill certain Medicare-reimbursed prescriptions. The pharmaceutical company allegedly identified certain patients in its free drug program for its vendor, and purportedly worked with the vendor to transfer the patients to the foundations, which received payments from the pharmaceutical company and then paid the copays for the Medicare patients. The allegations stem from a qui tam lawsuit, and the whistleblower will receive approximately $3.96 million of the recovery.[30]
B. Government Contracting and Procurement
- On July 22, a holding company agreed to pay $8 million to settle allegations that it violated the FCA by knowingly avoiding tariffs on imported brake parts. The government alleged that the holding company falsely improperly identified the brake parts as a type exempt from the tariffs. The whistleblowers, two former employees, will receive $1.48 million of the recovery.[31]
- On August 31, an engineering and construction firm and related entity agreed to pay approximately $5.6 million to resolve allegations that they violated the FCA and other civil claims by submitting inaccurate cost and labor hour estimates and certifications related to certain task orders for a federal contract with the U.S. Navy. The allegations stem from a qui tam lawsuit brought by a former employee, and the whistleblower’s share in the recovery was not disclosed at the time of the settlement announcement.[32]
- On September 10, an asphalt contractor agreed to pay more than $4.25 million over four years to resolve allegations that it misrepresented the materials it would use to pave federally-funded roads by falsely claiming that its asphalt mix contained a sufficient amount of binder to hold together and last a reasonable amount of time, in violation of the FCA.[33]
- On September 15, a software engineering firm that provides training systems to the Department of Defense agreed to pay more than $37 million in restitution to resolve allegations that the firm bribed an Air Force contracting official in exchange for procurement information. According to the government, the firm leveraged that information to secure government contracts for training simulators, causing a prime contractor to submit false invoices to the government. The firm paid the restitution as part of a broader plea agreement based on the same conduct, pursuant to which the firm pleaded guilty to conspiracy to commit wire fraud, but the civil settlement did not require admissions of liability. The majority owner, president and CEO of the firm separately agreed to pay $500,000 to resolve FCA allegations regarding his personal conduct.[34]
- On September 22, major federal construction contractors and a subsidiary admitted to improperly billing the Department of Energy for unreasonable and unallowable idle time in connection with a waste treatment plant project over a period of ten years, in violation of the FCA. Pursuant to the settlement, the companies agreed to pay $57.75 million and enter into a three-year corporate monitorship. Four whistleblowers will split $13.75 million.[35]
- On November 3, an Illinois-based charter school management company agreed to pay $4.5 million to settle allegations that it engaged in non-competitive bidding practices related to the Federal Communications Commission’s (“FCC”) E-Rate Program, thereby violating the FCA. The company allegedly rigged the bidding for E-Rate contracts between 2009 and 2012 so that its charter schools selected chosen technology vendors. The company’s chosen vendors also allegedly provided equipment at higher prices than FCC-approved prices for equipment with the same functionality. Finally, the company allegedly failed to maintain sufficient control over the FCC-reimbursed equipment, such that some of the equipment was missing. The company agreed to enter into a corporate compliance plan with the FCC.[36]
- On November 20, a federal contractor providing health care and IT services and solutions to federal agencies agreed to pay $18.98 million to settle allegations that it violated the FCA by using labor that did not meet requisite contractual qualifications and overcharging government agencies in connection with services provided under two General Services Administration (“GSA”) Multiple Award Schedule contracts. The federal contractor allegedly provided false information regarding its commercial discounting practices during contract negotiations with the government. The federal contractor investigated and disclosed the contractual violations to the United States, and received disclosure and cooperation credit.[37]
- On December 3, an ergonomic office furniture maker and its parent company agreed to pay $7.1 million to settle claims that they violated the FCA by overcharging the government for office furniture under a GSA contract. The government alleged that the company did not fulfill contractual obligations to provide GSA with accurate information about its sales practices during the contract negotiations, and the company also did not offer lower prices to government customers as required under the GSA contract. The allegations stem from a qui tam lawsuit brought by a former employee. The whistleblower will receive approximately $1.27 million of the recovery.[38]
- On December 17, a nationwide provider of electricity solutions for buildings and data centers agreed to pay $11 million to settle criminal and civil allegations relating to kickbacks and overcharges on federally-funded energy savings performance contracts. The provider agreed to pay $9.3 million to resolve allegations that it violated the FCA and AKS by soliciting and receiving over $2.5 million in kickbacks from subcontractors working on the contracts; including inflated estimates and improper costs in contract proposals; and overcharging federal agencies under the contracts. In a separate criminal settlement announced on the same day, the company admitted that it committed wire fraud when it fraudulently charged the government for design costs that it disguised and spread across various line items and also admitted that it violated the AKS when its former convicted employee solicited and received kickbacks from the subcontractors.[39]
III. LEGISLATIVE AND POLICY DEVELOPMENTS
A. Federal Legislative Developments
As we have reported previously, several COVID-19 related federal legislative developments in 2020—economic spending and stimulus packages—are likely to spur FCA enforcement. We have covered these developments in detail in updates throughout the COVID‑19 crisis (available here and here). The most notable legislation, the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”), marked the largest emergency stimulus package in history, providing $2.2 trillion worth of government funds to mitigate the effects of COVID-19.[40] The Act provides relief for businesses, industries, individuals, employers, and states in a number of ways, including a Small Business Administration (“SBA”) loan program offering up to $350 billion in relief, the Paycheck Protection Program (“PPP”), as well as economic stabilization programs to provide loans, loan guarantees, and funding for eligible industries, businesses, states, and municipalities.
In late December 2020, then President Trump signed a second massive stimulus bill, providing $900 billion of additional relief. Among other things, this new legislation renewed the PPP program, providing an additional $285 billion for additional loans for small businesses.[41] The new economic relief program tightened the funding terms and conditions in some respects, an effort apparently aimed at correcting some of the elements of the original program that were subject to criticism. The legislation caps new loans at $2 million, for example, and makes them available only to borrowers with fewer than 300 employees that experienced at least a 25% drop in sales from a year earlier in at least one quarter. In addition, publicly traded companies will not be eligible to apply for loans.
Before taking office on January 20, 2021, President Biden also announced a $1.9 trillion COVID relief plan that he aims to pass during his first 100 days in office.[42] Among other things, the plan provides $416 billion to launch a national vaccination program, $35 billion to make low-interest loans available to certain businesses, and sets aside another $1 trillion in additional stimulus checks for Americans.
There were no major developments with respect to federal FCA legislation in 2020. This may change soon, however. For example, in July, Senator Chuck Grassley (R-IA)—the original author of the FCA’s 1986 amendments—announced he is drafting legislation that would “clarify[y]” purported “ambiguities created by the courts” regarding the proper interpretation of the FCA.[43] In particular, Senator Grassley’s remarks highlighted his concerns about DOJ’s authority to dismiss FCA cases despite relators’ objections, as well as DOJ’s practice of increasingly exercising that authority following DOJ’s issuance of the Granston Memo, on which we have reported previously. We will closely monitor this and other developments at the federal level in the coming year.
B. COVID-19 Enforcement Policy
Under the outgoing administration, DOJ focused on preventing and punishing COVID-19-related fraud. To date, DOJ has scrutinized several aspects of the stimulus funding under the CARES Act, in particular, such as in connection with certifications of compliance with loan program requirements, as well as submission of false claims allegedly kickback-tainted, medically unnecessary, and/or otherwise not provided as represented.[44]
This policy played out in 2020, with DOJ officials announcing plans to “deploy the [FCA] against those who commit fraud related to the various COVID-19 stimulus programs,” particularly the Provider Relief Fund (“PRF”) and the Paycheck Protection Program—funding programs put into place by the CARES Act. These programs, which impose numerous requirements on funding recipients, make available significant sums of money that DOJ considers may provide “a number of opportunities for fraud.”[45]
The Biden administration will almost certainly continue to focus on COVID-19 enforcement. What other enforcement changes or priorities come from the Biden administration remain to be seen.
C. State Legislative Developments
As an incentive for seeking HHS-OIG approval of their false claims act statutes, states can receive “a 10-percentage-point increase in their share of any amounts” recovered under the relevant laws.[46] To receive approval, state statutes must (among other requirements) contain provisions that are “at least as effective in rewarding and facilitating qui tam actions” as those in the federal FCA, and must contain civil penalties at least equivalent to those imposed by the federal FCA.[47] A similar requirement is that a given state’s statute must provide for civil penalty increases “at the same rate and time as those authorized under the [federal] FCA” pursuant to the Federal Civil Penalties Inflation Adjustment Act Improvements Act of 2015.[48]
Currently, the total number of states with approved statutes stands at twenty-one (California, Colorado, Connecticut, Delaware, Georgia, Hawaii, Illinois, Indiana, Iowa, Massachusetts, Montana, Nevada, New York, North Carolina, Oklahoma, Rhode Island, Tennessee, Texas, Vermont, Virginia, and Washington). Eight states have laws that have not yet been deemed to meet the federal standards (Florida, Louisiana, Michigan, Minnesota, New Hampshire, New Jersey, New Mexico, and Wisconsin).[49] Thirty-one states have enacted some version of the False Claims Act.[50
Several jurisdictions also enacted or advanced false claims act legislation in 2020. In the District of Columbia, the D.C. Council enacted legislation amending the District’s existing false claims act (D.C. Code Ann. § 2-381.01 et seq.) to expressly authorize tax-related false claims actions against persons who “reported net income, sales, or revenue totaling $1 million or more in a tax filing to which [the relevant] claim, record, or statement pertained, and the damages pleaded in the action total $350,000 or more.”[51] The bill authorizes treble damages for tax-related violations, meaning District taxpayers could be liable for three times the amount not only of any taxes, but also of any interest and tax penalties.[52] Because the District’s existing false claims statute excluded tax-related claims from false claims liability, the new legislation represents a major policy shift.[53] In amending its false claims statute in this fashion, the District joins Illinois and New York as jurisdictions that provide for tax-related FCA liability.[54]
In Pennsylvania, which has no statute analogous to the FCA, the legislature advanced a false claims act bill that would enable private citizens to bring lawsuits on behalf of the state against anyone who “[k]nowingly presents or causes to be presented a false or fraudulent claim for payment or approval” or “[k]nowingly makes, uses or causes to be made or used, a false record or statement material to a false or fraudulent claim.”[55] The bill would also require the Attorney General to make recommendations to state agencies on how to prevent false claims violations from occurring.[56] The new law would empower the Pennsylvania Office of the Attorney General to enforce its provisions, including via civil investigative demands.[57] The bill largely mirrors the FCA and was first referred to the House Human Services Committee on May 21, 2020.[58] In September 2020, the House committee approved an amended bill to include limited civil liability protections for entities that follow all state and federal directives regarding COVID-19, along with civil fraud provisions matching federal law.[59] To date, the bill is awaiting a vote in the Pennsylvania General Assembly.
We also reported in our 2020 Mid-Year Update on a bill passed by the California Assembly, Assembly Bill No. 1270, which would alter the state’s false claim act considerably, including by amending the act to limit the definition of materiality to include only “the potential effect” of an alleged false record or statement “when it is made,” without consideration—contrary to the U.S. Supreme Court’s 2016 decision in Universal Health Services v. United States ex rel. Escobar[60]—of “the actual effect of the false record or statement when it is discovered.”[61] The amendments would also extend the act to tax-related cases where the damages pleaded exceed $200,000 and a defendant’s state-taxable income or sales exceed $500,000.[62] After the bill stalled in the State Senate, a California Assembly member (Mark Stone, D-Monterey Bay) introduced a substantially similar bill, Assembly Bill No. 2570.[63] As with its predecessor, AB-2570 stalled in the State Senate in 2020.
IV. NOTABLE CASE LAW DEVELOPMENTS
The second half of 2020 saw a number of important case law developments, including with respect to falsity, materiality, and the FCA’s important threshold bars. We cover the most notable cases below.
A. A Circuit Split Over “Objective Falsity” Progresses to the Supreme Court
As discussed in our Mid-Year Update, the issue of whether and when differences in physician medical opinions may satisfy the FCA’s “falsity” element is driving critical developments in FCA jurisprudence. In particular, a circuit split emerged after the Eleventh Circuit’s decision in United States v. AseraCare, Inc., in which the court held that claims cannot be “deemed false” under the FCA based solely on “a reasonable disagreement between medical experts” as to a medical provider’s clinical judgment. 938 F.3d 1278, 1281 (11th Cir. 2019). By contrast, the Third Circuit held in United States ex rel. Druding v. Care Alternatives that a “physician’s judgment may be scrutinized and considered ‘false’” and that a “difference of medical opinion is enough evidence to create a triable dispute of fact regarding FCA falsity.” 952 F.3d 89, 100–01 (3d Cir. 2020). The Ninth Circuit reached a similar result in Winter ex rel. United States v. Gardens Regional Hospital and Medical Center, holding that an FCA claim based on an alleged lack of medical necessity may suffice to survive a motion to dismiss. 953 F.3d 1108, 1117 (9th Cir. 2020).
In September 2020, Care Alternatives petitioned the Supreme Court for a writ of certiorari to challenge the Third Circuit’s rejection of the AseraCare “objective falsity” standard. Specifically, Care Alternatives asked the Court to decide “[w]hether a physician’s honestly held clinical judgment regarding hospice certification can be ‘false’ under the False Claims Act based solely on a reasonable difference of opinion among physicians.” Pet. for Writ of Cert., Care Alternatives v. United States, et al., No. 20-371 (U.S. Sept. 16, 2020).
In its petition, Care Alternatives contended that the Third Circuit’s recent decision created a “square split” with the Eleventh Circuit’s AseraCare decision “on an issue of critical importance to the millions of Americans who require hospice care annually and the thousands of hospices and physicians who provide that care.” Id. at 1–2. Care Alternatives also argued that the Third Circuit’s rejection of an objective falsity standard “opens up hospices and physicians to crushing financial liability and reputational harm, notwithstanding near universal acknowledgment that determinations about life expectancy are notoriously difficult and inexact.” Id. at 2. Further, it highlighted the “untenable prospect . . . that hospices in New Jersey [because of the Third Circuit’s decision] will face treble damages for the same difficult medical judgments that cannot be second-guessed in Florida,” in light of the Eleventh Circuit’s AseraCare case. Id. at 3.
Given the stakes, the case has attracted attention from industry participants. After Care Alternatives filed its petition, two groups submitted amicus briefs: one by a group of Hospice, Health Care, and Physician Organizations, and the other from the Chamber of Commerce of the United States of America and the Pharmaceutical Research and Manufacturers of America (“PhRMA”). See Br. for the Hospice, Health Care, and Physician Organizations as Amici Curiae, Care Alternatives v. United States, et al., No. 20-371 (U.S. Oct. 23, 2020) (“Hospice Brief”); Br. of Chamber of Commerce of the United States et al. as Amici Curiae, Care Alternatives v. United States, et al., No. 20-371 (U.S. Oct. 23, 2020) (“Chamber of Commerce Brief”). The briefs highlighted the risks the Third Circuit’s decision poses for providers and for recipients of government benefits more broadly (such as government contractors). See generally Hospice Brief; Chamber of Commerce Brief. The amici likewise cited the broader developing circuit split over “objective falsity” as another reason why the Court should grant Care Alternatives’ petition. Chamber of Commerce Brief at 8–10.
B. Courts Continue to Grapple with the FCA’s Materiality and Scienter Requirements Post-Escobar
In the latter half of 2020, federal appellate courts continued to weigh in on the critical issues of materiality and scienter under the FCA in the wake of the Supreme Court’s seminal decision in Universal Health Services v. United States ex rel. Escobar, 136 S. Ct. 1989 (2016). The Court’s clear directive in Escobar was that courts should scrutinize whether plaintiffs have alleged facts sufficient to satisfy the “rigorous” and demanding materiality standard the FCA imposes. See id. at 2004 n.6 (rejecting the notion that materiality cannot be decided at the pleadings stage). Two Circuit Courts of Appeals took up this task in notable ways in the latter half of 2020.
First, in United States v. Strock, the Second Circuit considered what counts as a “payment decision” for purposes of assessing materiality under a fraudulent inducement theory of FCA liability. 982 F.3d 51 (2d Cir. Dec. 3, 2020). Under a fraudulent inducement theory, “FCA liability attaches not because a defendant has submitted any claim for payment that is ‘literally false,’ but instead because ‘the contract under which payment [is] made is procured by fraud.’” Id. at 60 (quoting United States ex rel. Longhi v. United States, 575 F.3d 458, 467–68 (5th Cir. 2009)). In Strock, the court evaluated whether Escobar materiality analysis applied to the government’s initial decision to award the contract, the government’s subsequent decision to pay claims under the contract, or both. The government alleged that a putatively service-disabled veteran-owned small business (“SDVOSB”) was used “as a front to funnel [government] contract work” to another contractor. Id. at 56. The U.S. District Court for the Western District of New York granted the defendants’ motion to dismiss and concluded that Escobar only required a showing of materiality in connection with the government’s initial awarding of the contract. Id. at 58–60.
On appeal, the Second Circuit reversed the district court’s dismissal of the FCA claims against one individual defendant and vacated the district court’s dismissal of the FCA claims against the corporate defendant under a vicarious liability theory. The Second Circuit reasoned that the nature of fraudulent inducement cases required it to assign the meaning of “payment decisions” subject to Escobar analysis a “broader scope” than the lower court had. Id. at 60. The Second Circuit interpreted both the government’s initial contract award and subsequent payments of claims as “payment decisions” requiring a materiality analysis under Escobar. Id. at 59–60.
Earlier in 2020, the Fifth Circuit in United States ex rel. Porter v. Magnolia Health Plan, Inc. also applied Escobar’s materiality standard to a case decided at the pleadings stage. 810 F. App’x 237 (5th Cir. 2020). There, a registered nurse alleged that her former employer violated the FCA by staffing care and case manager positions with licensed practical nurses in contravention of contractual requirements. The district court dismissed the FCA claims, concluding that “broad boilerplate language generally requiring a contractor to follow all laws” was “too general to support a FCA claim.” Id. at 242. In affirming, the Fifth Circuit agreed that the applicable contracts did not require the defendant to staff relevant positions with registered nurses and that the boilerplate language was not sufficient to establish an FCA claim. The Fifth Circuit also explained that the “continued payments to and contracts with” the defendant “substantially increase the burden . . . in establishing materiality,” which the plaintiff did not meet. Id. Specifically, the Fifth Circuit noted that “the Mississippi Division of Medicaid took no action after Plaintiff-Appellant informed the Division” of this alleged misconduct but rather “continued payment and renewed its contract with [the former employer] several times.” Id. Even after the plaintiff’s suit was unsealed, the third-party Medicaid contractor awarded the plaintiff’s former employer “a contract for the fourth time.” Id. The Fifth Circuit also affirmed the district court’s dismissal with prejudice, finding “no reasonable basis to predict that [the plaintiff] c[ould] recover on her claims” and that any amendment of the nurse’s complaint thus would be futile, in part, because of the government’s continued payments and contracting arrangements with the nurse’s former employer. Id. at 243.
On December 9, 2020, after the Fifth Circuit refused to rehear the case, the relator petitioned for a writ of certiorari, asking the Supreme Court to clarify to what extent Escobar altered the Rule 12(b)(6) plausibility standard the Court imposed in Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) and Ashcroft v. Iqbal, 556 U.S. 662 (2009). Pet. for Writ of Cert., United States ex rel. Porter v. Magnolia Health Plan, Inc., No. 20-786 (U.S. Dec. 9, 2020). Specifically, the petition asked the Court to decide “whether the Supreme Court ruling in Escobar overruled or modified the standard of review to be used in ruling upon Rule 12(b)(6) motions to dismiss in cases involving the False Claims Act so as to require ‘proof’ or ‘evidence’ at the initial pleading stage above and beyond the plausibility standard set forth in Twombly and Iqbal.” Id. at iii. The Court denied the qui tam plaintiff’s petition on January 19, 2021.
C. Courts Continue to Scrutinize DOJ’s Discretion to Dismiss Qui Tam Claims
1. A Third Standard for DOJ’s Dismissal Authority?
In the wake of the 2018 Granston Memo, which instructed DOJ attorneys to consider dismissal of a qui tam case when recommending declination, DOJ has more regularly invoked its dismissal authority under 31 U.S.C. § 3730(c)(2)(A) than it did in for decades previously. Historically, courts have split based on whether they follow the Ninth Circuit’s Sequoia Orange test or the D.C. Circuit’s Swift test. Under the Sequoia Orange approach, the government may dismiss a qui tam case if: (1) it identifies a valid government purpose; (2) a rational relation exists between the dismissal and the accomplishment of that purpose; and (3) dismissal is not fraudulent, arbitrary and capricious, or illegal. United States ex rel. Sequoia Orange Co. v. Baird-Neece Packing Corp., 151 F.3d 1139, 1145 (9th Cir. 1998). The Swift test, by contrast, affords the government an “unfettered” right to dismiss a case such that the decision is “unreviewable” except in instances of “fraud on the court.” Swift v. United States, 318 F.3d 250, 252–53 (D.C. Cir. 2003). Both standards generally favor the government’s discretion, albeit to different degrees, and DOJ regularly argues in its motions to dismiss that it has sufficient discretion to dismiss a case under either standard.
This past August, the Seventh Circuit suggested that the split may have little practical significance. In United States ex rel. CIMZNHCA, LLC v. UCB, Inc., 970 F.3d 835 (7th Cir. 2020), the court reviewed a district court’s denial of the government’s attempt to dismiss the case, which concerned the alleged provision of kickbacks to physicians for prescriptions of a drug used to treat Crohn’s disease. Id. at 839. In moving for dismissal, the government argued that the allegations “lack[ed] sufficient merit to justify the cost of investigation and prosecution and [were] otherwise . . . contrary to the public interest.” Id. at 840. But the district court, applying the Sequoia Orange standard, deemed the government’s decision “arbitrary and capricious” and “not rationally related to a valid governmental purpose.” Id. (internal quotation marks omitted).
The Seventh Circuit reversed, calling the choice between the Sequoia Orange and Swift standards “a false one, based on a misunderstanding of the government’s rights and obligations under the False Claims Act.” Id. at 839. Instead, the court viewed the government’s motion as a motion to intervene and dismiss and held that Federal Rule of Civil Procedure 41 (which governs voluntary dismissal by plaintiffs generally) supplied “the beginning and end of [the court’s] analysis.” Id. at 849. While Rule 41(a)(1)(A) states that the voluntary dismissal right is “[s]ubject to . . . any applicable federal statute,” the “only authorized statutory deviation from Rule 41” in the FCA itself is the requirement that a relator be given notice and an opportunity to be heard in the event that the government seeks to dismiss the case over the relator’s objection. See id. at 850. The court acknowledged that such a hearing may amount to little more than formality in cases where there are no questions about the propriety of the government’s exercise of its dismissal authority; but the court noted that Rule 41’s conditions on the timing of voluntary dismissal motions could arise in Section 3730(c)(2)(A) hearings in cases where “the government’s chance to serve notice of dismissal has passed . . . and the relator . . . refuses to agree to dismissal.” Id. at 850–51.
Turning to the Sequoia Orange and Swift standards, the court held that Sequoia Orange simply means that dismissal “may not violate the substantive component of the Due Process Clause,” id. at 851, which the court characterized as a “bare rationality standard” targeting “only the most egregious official conduct” that “shocks the conscience” or “offend[s] even hardened sensibilities,” id. at 852 (internal quotation marks omitted) (alteration in original). The court found that the government’s dismissal decision, based as it was on the fact that agency guidance and rules had repeatedly “held that the conduct complained of is probably lawful,” did not rise to this level. See id. At the same time, the court rejected the idea that the relatively formal nature of Section 3730(c)(2)(A) hearings “justif[ies] imposing on the government in each case the burden of satisfying Sequoia Orange’s ‘two-step test’ before the burden is put back on the relator to show unlawful executive conduct.” Id. at 853.
In sum, while the court recognized the value of a Sequoia Orange-type standard focused on the outer constitutional limits on the exercise of the government’s prosecutorial discretion, the court’s holding suggested that it believes that limit lies closer to the even‑more‑forgiving Swift standard than to the “two-step” approach set forth in Sequoia Orange. The Seventh Circuit seems to have believed that the district court lost sight of the constitutional underpinnings of the “rational basis” test—and that a focus on the procedural parameters of Rule 41 can help avoid this error, insofar as they are consistent with a very forgiving approach to the government’s exercise of its dismissal authority. Accordingly, going forward we may well see DOJ intervene for the purposes of dismissal to exercise its (c)(2)(A) dismissal authority more often, at least in Seventh Circuit courts.
2. The Ninth Circuit Explores Limits on the Appealability of Denials of the Government’s Motions to Dismiss Under Section 3730(C)(2)(A)
In another notable case regarding DOJ’s dismissal authority, the Ninth Circuit issued a decision that could create more pressure for DOJ, when it wishes to dismiss a case, to intervene in the action first. In United States v. Academy Mortgage Corp., 968 F.3d 996 (9th Cir. 2020), the district court denied DOJ’s motion to dismiss on the ground that the government’s cost-benefit justification was insufficient to satisfy the Sequoia Orange standard. Id. at 1001. The government had claimed that discovery would be burdensome, but the court believed that the government had an incomplete understanding of the potential monetary recovery in the case given the limited nature of the government’s investigation. Id. The government appealed the denial of its motion under the collateral order doctrine, rather than seek to have the issue certified for interlocutory review. See id.
The Ninth Circuit dismissed the appeal for lack of jurisdiction, holding that the collateral order doctrine does not apply to denials of motions to dismiss under Section 3730(c)(2)(A), “at least in cases where the Government has not exercised its right to intervene.” Id. at 1005. Citing the government’s professed concern regarding discovery burdens, the court reasoned that, when the government has not intervened in a qui tam action, it is not a party to the action and its discovery obligations accordingly are the same as those of any other non‑party under Federal Rule of Civil Procedure 45. Id. at 1006. The court noted that the path to appellate review of a question of discovery burdens on a third party typically is to defy a subpoena and appeal the resulting contempt citation; orders merely denying motions to quash under Rule 45 “generally cannot be immediately appealed under the collateral order doctrine.” Id. at 1006–07. The court stated the core of its concern as follows: “It would be incongruous to hold, as we are asked to do here, that the Government’s interest in dismissing the case to avoid the possibility of future onerous discovery requests is important enough to merit an immediate appeal, when third parties actually faced with burdensome subpoenas have no such right.” Id. at 1007 (emphases in original). Although the court stated that the government could pursue interlocutory review, the court’s opinion could be read to suggest that the case does not present a “controlling question of law as to which there is substantial ground for difference of opinion” where the government’s rationale for dismissal is a mere “run-of-the-mill litigation burden[].” Id. at 1009.
The courts in both Academy Mortgage and UCB treated the question of DOJ’s intervention as affecting which legal framework should apply to the analysis of DOJ’s dismissal authority. Practically speaking, that reasoning may encourage DOJ to intervene in cases in which it otherwise would not seek to do so, for the limited purpose of strengthening its posture in moving to dismiss the case.
D. Developments on the First-to-File Bar and Res Judicata
Under Section 3730(b)(5) of the FCA, “[w]hen a person brings an [FCA] action . . . no person other than the Government may intervene or bring a related action based on the facts underlying the pending action.” 31 U.S.C. § 3730(b)(5). The Circuits have split over whether this “first-to-file bar” is jurisdictional. The First, Second, and D.C. Circuits have held that the bar is not jurisdictional, whereas the Fourth, Fifth, Sixth, Ninth, and Tenth Circuits have concluded that the bar is a matter of courts’ subject‑matter jurisdiction. See In re Plavix Marketing, Sales Practices & Products Liability Litig., 974 F.3d 228, 232 (3d Cir. 2020) (collecting cases).
In a September 1, 2020 opinion, the Third Circuit joined the former camp, relying primarily on the “clear statement rule”: “As the Supreme Court has recently instructed, unless Congress states clearly that a rule is jurisdictional, we will treat it as nonjurisdictional. . . . [Defendants] point to no language in § 3730(b)(5), nor do we see any, that ‘plainly show[s] that Congress imbued [the first-to-file] bar with jurisdictional consequences.’” Id. at 232 (second and third alterations in original) (citation omitted). The court also rejected the defendants’ argument that the bar is a matter of constitutional standing, concluding instead that it “asks only ‘whether [the relator] falls within the class of plaintiffs whom Congress has authorized to sue,’ which is another way to ask whether the statute gives it a cause of action.” Id. (alteration in original) (citation omitted). Accordingly, a motion to dismiss under the first‑to‑file bar “falls under Rule 12(b)(6) for failure to state a claim.” Id. at 233.
In a separate case, the State of New Mexico filed a complaint in state court while the Plavix litigation was pending but after the State declined to intervene in that litigation. See State ex rel. Balderas v. Bristol-Myers Squibb Co., 436 P.3d 724, 727 (N.M. Ct. App. 2018). The state trial and appellate courts held that the dismissal of the Plavix relator’s claims with prejudice did not act as dismissal with prejudice as to the government. Id. at 734. The court cited favorably to other decisions reasoning that a non-intervention decision does not automatically mean the government does not see merit in the case in question, and that “perverse incentives” would arise if dismissal with prejudice as to a relator also precluded claims by the government. Id. at 731. For example, the government essentially would have to intervene in every case simply to protect its ability to sue a defendant later, id., which would defeat the purpose of statutory provisions granting the government discretion to intervene.
The defendants filed a petition in the Supreme Court for a writ of certiorari in early September, a request which remains pending. See generally Pet. for Writ of Cert., State ex rel. Balderas v. Bristol-Myers Squibb Co., No. 20-293 (U.S. Sept. 3, 2020). If the Court takes the case, it will be an opportunity to resolve a Circuit split over whether the government is bound by with-prejudice dismissals of qui tam complaints. The Fifth and Eleventh Circuits have answered that question in the negative, the Seventh and Ninth Circuits in the affirmative. See id. at 13–19.
E. The D.C. Circuit Affirms an Award of Summary Judgment Where Defendant Failed to Adequately Address Government’s Legal Theories
It is difficult for any plaintiff to prevail on a motion for summary judgment. This is particularly so in FCA actions, which demand that plaintiffs prove various rigorously construed and fact‑intensive elements, including materiality and scienter.
In August 2016, however, the U.S. District Court for the District of Columbia granted the government’s motion for summary judgment in a case against a home health care company alleged to have submitted claims for reimbursement to the District of Columbia Medicaid Program for services that purportedly lacked adequate documentation. United States v. Dynamic Visions Inc., 220 F. Supp. 3d 16, 22 (D.D.C. 2016).
The district court’s opinion is notable given how rarely these motions are granted. Just as noteworthy is the fact that, in August 2020, the D.C. Circuit largely affirmed the lower court’s award of summary judgment in the government’s favor. United States v. Dynamic Visions Inc, 971 F.3d 330, 338–40 (D.C. Cir. 2020). The D.C. Circuit highlighted that, on appeal, the defendant-appellant had failed to meaningfully address the government’s theory that patients had inadequate “plan of care” documentation in several different regards, having chosen instead to “respond[] only with highly generalized statements to the effect that they submitted plans of care for Medicaid recipients signed by their physicians, . . . that they maintained a policy and procedure manual that was compliant with [Department of Health Care Finance] regulations[,] and [that they] followed the policy and procedures stated in the manual.” Id. at 337 (internal quotation marks omitted). Because the defendant-appellant failed to provide supporting evidence for those assertions—namely, the manual in question—the court held that “[t]hose statements are too conclusory to create a genuine issue.” Id.
V. CONCLUSION
As always, Gibson Dunn will continue to monitor these developments and others in the FCA space and stands ready to answer any questions you may have. We will report back to you on the latest news mid-year, in July 2021.
____________________
[1] See U.S. Dep’t of Justice, Memorandum from Rachel Brand, Associate Attorney General (Nov. 16, 2017), https://www.justice.gov/opa/press-release/file/1012271/download.
[2] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Justice Department Recovers Over $2.2 Billion from False Claims Act Cases in Fiscal Year 2020 (Jan. 14, 2021), https://www.justice.gov/opa/pr/justice-department-recovers-over-22-billion-false-claims-act-cases-fiscal-year-2020 [hereinafter DOJ FY 2020 Recoveries Press Release].
[3] See U.S. Dep’t of Justice, Fraud Statistics Overview (Jan. 14, 2021), https://www.justice.gov/opa/press-release/file/1354316/download [hereinafter DOJ FY 2020 Stats].
[4] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Remarks of Deputy Assistant Attorney General Michael D. Granston at the ABA Civil False Claims Act and Qui Tam Enforcement Institute (Dec. 2, 2020), https://www.justice.gov/opa/speech/remarks-deputy-assistant-attorney-general-michael-d-granston-aba-civil-false-claims-act.
[5] See DOJ FY 2020 Stats.
[6] See Press Release, U.S. Atty’s Office for the W. Dist. Of Ky., California Genetic Testing Company Agrees To Pay $8.25 Million To Resolve False Claims Allegations; Paducah, Ky, Area Hospital Also Settles (July 1, 2020), https://www.justice.gov/usao-wdky/pr/california-genetic-testing-company-agrees-pay-825-million-resolve-false-claims.
[7] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Novartis Pays Over $642 Million to Settle Allegations of Improper Payments to Patients and Physicians (July 1, 2020), https://www.justice.gov/opa/pr/novartis-pays-over-642-million-settle-allegations-improper-payments-patients-and-physicians; Press Release, U.S. Atty’s Office for the S. Dist. of N.Y., Acting Manhattan U.S. Attorney Announces $678 Million Settlement Of Fraud Lawsuit Against Novartis Pharmaceuticals For Operating Sham Speaker Programs Through Which It Paid Over $100 Million To Doctors To Unlawfully Induce Them To Prescribe Novartis Drugs (July 1, 2020), https://www.justice.gov/usao-sdny/pr/acting-manhattan-us-attorney-announces-678-million-settlement-fraud-lawsuit-against.
[8] See Press Release, U.S. Atty’s Office for the Middle Dist. of Fla., Hope Hospice Agrees To Pay $3.2 Million To Settle False Claims Act Liability (July 8, 2020), https://www.justice.gov/usao-mdfl/pr/hope-hospice-agrees-pay-32-million-settle-false-claims-act-liability.
[9] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Oklahoma City Hospital, Management Company, And Physician Group To Pay $72.3 Million To Settle Federal And State False Claims Act Allegations Arising From Improper Payments To Referring Physicians (July 8, 2020), https://www.justice.gov/opa/pr/oklahoma-city-hospital-management-company-and-physician-group-pay-723-million-settle-federal.
[10] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Universal Health Services, Inc. And Related Entities To Pay $122 Million To Settle False Claims Act Allegations Relating To Medically Unnecessary Inpatient Behavioral Health Services And Illegal Kickbacks (July 10, 2020), https://www.justice.gov/opa/pr/universal-health-services-inc-and-related-entities-pay-122-million-settle-false-claims-act.
[11] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Twenty-Seven Skilled Nursing Facilities Controlled By Longwood Management Corporation To Pay $16.7 Million To Resolve False Claims Act Allegations (July 13, 2020), https://www.justice.gov/opa/pr/twenty-seven-skilled-nursing-facilities-controlled-longwood-management-corporation-pay-167.
[12] See Press Release, U.S. Atty’s Office for the W. Dist. of Wash., DOJ settles False Claims Act allegations against drug testing lab with operations in Tacoma and Denver (July 20, 2020), https://www.justice.gov/usao-wdwa/pr/doj-settles-false-claims-act-allegations-against-drug-testing-lab-operations-tacoma-and.
[13] See Press Release, U.S. Atty’s Office for the S. Dist. of N.Y., Acting Manhattan U.S. Attorney Announces $49 Million Settlement With Biotech Testing Company For Fraudulent Billing And Kickback Practices (July 23, 2020), https://www.justice.gov/usao-sdny/pr/acting-manhattan-us-attorney-announces-49-million-settlement-biotech-testing-company.
[14] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Indivior Solutions Pleads Guilty To Felony Charge And Indivior Entities Agree To Pay $600 Million To Resolve Criminal And Civil Investigations As Part Of DOJ’s Largest Opioid Resolution (July 24, 2020), https://www.justice.gov/opa/pr/indivior-solutions-pleads-guilty-felony-charge-and-indivior-entities-agree-pay-600-million.
[15] See Press Release, U.S. Atty’s Office for the Dist. of N.J., Pharmaceutical Company Agrees to Pay $3.5 Million to Resolve Allegations of Violating False Claims Act (July 28, 2020), https://www.justice.gov/usao-nj/pr/pharmaceutical-company-agrees-pay-35-million-resolve-allegations-violating-false-claims.
[16] See Press Release, U.S. Atty’s Office for the E. Dist. of N.Y., New York Hospice Provider Settles Civil Healthcare Fraud Allegations (Aug. 19, 2020), https://www.justice.gov/usao-edny/pr/new-york-hospice-provider-settles-civil-healthcare-fraud-allegations.
[17] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, DUSA Pharmaceuticals To Pay U.S. $20.75 Million To Settle False Claims Act Allegations Relating To Promotion Of Unsupported Drug Administration Process (Aug. 24, 2020), https://www.justice.gov/opa/pr/dusa-pharmaceuticals-pay-us-2075-million-settle-false-claims-act-allegations-relating.
[18] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, West Virginia Hospital Agrees To Pay $50 Million To Settle Allegations Concerning Improper Compensation To Referring Physicians (Sept. 9, 2020), https://www.justice.gov/opa/pr/west-virginia-hospital-agrees-pay-50-million-settle-allegations-concerning-improper.
[19] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, William M. Kelly, M.D., Inc And Omega Imaging, Inc. Agree To Pay $5 Million To Resolve Alleged False Claims For Unsupervised And Unaccredited Radiology Services (Sept. 9, 2020), https://www.justice.gov/opa/pr/william-m-kelly-md-inc-and-omega-imaging-inc-agree-pay-5-million-resolve-alleged-false-claims.
[20] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, The Scripps Research Institute To Pay $10 Million To Settle False Claims Act Allegations Related To Mischarging NIH-Sponsored Research Grants (Sept. 11, 2020), https://www.justice.gov/opa/pr/scripps-research-institute-pay-10-million-settle-false-claims-act-allegations-related.
[21] See Press Release, U.S. Atty’s Office for the S. Dist. of N.Y., Acting Manhattan U.S. Attorney Announces $11.5 Million Settlement With Biotech Testing Company For Fraudulent Billing And Kickback Practices (Sept. 22, 2020), https://www.justice.gov/usao-sdny/pr/acting-manhattan-us-attorney-announces-115-million-settlement-biotech-testing-company.
[22] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Gilead Agrees To Pay $97 Million To Resolve Alleged False Claims Act Liability For Paying Kickbacks (Sept. 23, 2020), https://www.justice.gov/opa/pr/gilead-agrees-pay-97-million-resolve-alleged-false-claims-act-liability-paying-kickbacks.
[23] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Lakeway Regional Medical Center LLC And Co-Defendants Agree To Pay Over $15.3 Million To Resolve Allegations They Fraudulently Obtained Government-Insured Loan And Misused Loan Funds (Sept. 28, 2020), https://www.justice.gov/opa/pr/lakeway-regional-medical-center-llc-and-co-defendants-agree-pay-over-153-million-resolve.
[24] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Medical Device Maker Merit Medical To Pay $18 Million To Settle Allegations Of Improper Payments To Physicians (Oct. 14, 2020), https://www.justice.gov/opa/pr/medical-device-maker-merit-medical-pay-18-million-settle-allegations-improper-payments.
[25] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Justice Department Announces Global Resolution of Criminal and Civil Investigations with Opioid Manufacturer Purdue Pharma and Civil Settlement with Members of the Sackler Family (Oct. 21, 2020), https://www.justice.gov/opa/pr/justice-department-announces-global-resolution-criminal-and-civil-investigations-opioid.
[26] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Medtronic to Pay Over $9.2 Million To Settle Allegations of Improper Payments to South Dakota Neurosurgeon (Oct. 29, 2020), https://www.justice.gov/opa/pr/medtronic-pay-over-92-million-settle-allegations-improper-payments-south-dakota-neurosurgeon.
[27] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Medicare Advantage Provider to Pay $6.3 Million to Settle False Claims Act Allegations (Nov. 16, 2020), https://www.justice.gov/opa/pr/medicare-advantage-provider-pay-63-million-settle-false-claims-act-allegations.
[28] See Press Release, U.S. Atty’s Office for the E. Dist. of Pa., Former Owners of Therakos, Inc. Pay $11.5 Million to Resolve False Claims Act Allegations of Promotion of Drug-Device System for Unapproved Uses to Pediatric Patients (Nov. 19, 2020), https://www.justice.gov/usao-edpa/pr/former-owners-therakos-inc-pay-115-million-resolve-false-claims-act-allegations.
[29] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Home Health Agency and Former Owner to Pay $5.8 Million to Settle False Claims Act Allegations (Nov. 20, 2020), https://www.justice.gov/opa/pr/home-health-agency-and-former-owner-pay-58-million-settle-false-claims-act-allegations.
[30] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Biogen Agrees To Pay $22 Million To Resolve Alleged False Claims Act Liability For Paying Kickbacks (Dec. 17, 2020), https://www.justice.gov/opa/pr/biogen-agrees-pay-22-million-resolve-alleged-false-claims-act-liability-paying-kickbacks.
[31] See Press Release, U.S. Atty’s Office for the E. Dist. of Mich., CWD Holdings To Pay $8 Million To Resolve False Claims Act Allegations Relating To Unpaid Import Duties (July 22, 2020), https://www.justice.gov/usao-edmi/pr/cwd-holdings-pay-8-million-resolve-false-claims-act-allegations-relating-unpaid-import.
[32] See Press Release, U.S. Atty’s Office for the E. Dist. of Va., CDM Smith and CDM Federal Programs Agrees to $5.6 Million Settlement (Aug. 31, 2020), https://www.justice.gov/usao-edva/pr/cdm-smith-and-cdm-federal-programs-agrees-56-million-settlement.
[33] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Asphalt Contractor To Pay $4.25 Million To Settle Claims That It Misled The Government As To The Materials Used To Pave Road (Sept. 10, 2020), https://www.justice.gov/opa/pr/asphalt-contractor-pay-425-million-settle-claims-it-misled-government-materials-used-pave.
[34] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Quantadyn Corporation And Owner Settle False Claims Act Allegations of Bribery To Obtain Government Contracts For Simulators (Sept. 15, 2020), https://www.justice.gov/opa/pr/quantadyn-corporation-and-owner-settle-false-claims-act-allegations-bribery-obtain-government.
[35] See Press Release, U.S. Atty’s Office for the E. Dist. of Wash., Bechtel & Aecom, U.S. Department of Energy (DOE) Contractors, Agree to Pay $57.75 Million to Resolve Claims of Time Charging Fraud at Doe’s Hanford Waste Treatment Plant (Sept. 22, 2020), https://www.justice.gov/usao-edwa/pr/bechtel-aecom-us-department-energy-doe-contractors-agree-pay-5775-million-resolve-0.
[36] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Illinois-Based Charter School Management Company To Pay $4.5 Million To Settle Claims Relating To E-Rate Contracts (Nov. 3, 2020), https://www.justice.gov/opa/pr/illinois-based-charter-school-management-company-pay-45-million-settle-claims-relating-e-rate.
[37] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Federal Contractor Agrees to Pay $18.98 Million for Alleged False Claims Act Caused by Overcharges and Unqualified Labor (Nov. 20, 2020), https://www.justice.gov/opa/pr/federal-contractor-agrees-pay-1898-million-alleged-false-claims-act-caused-overcharges-and.
[38] See Press Release, Office of Pub. Affairs, U.S. Dep’t of Justice, Workrite Companies to Pay $7.1 Million to Settle Alleged Furniture Overcharges (Dec. 3, 2020), https://www.justice.gov/opa/pr/workrite-companies-pay-71-million-settle-alleged-furniture-overcharges.
[39] See Press Release, U.S. Atty’s Office for the Dist. of Vt., Government Contractor Admits Scheme to Inflate Costs on Federal Projects and Pays $11 Million to Resolve Criminal and Civil Probes (Dec. 17, 2020), https://www.justice.gov/usao-vt/pr/government-contractor-admits-scheme-inflate-costs-federal-projects-and-pays-11-million.
[40] See Gibson, Dunn, & Crutcher LLP, Emergency Federal Measures to Combat Coronavirus (Mar. 18, 2020), https://www.gibsondunn.com/emergency-federal-measures-to-combat-coronavirus/.
[41] Consolidated Appropriations Act, 2021, Pub. L. No. 116-159, 116th Cong. (2019-2020).
[42] Build Back Better, President-elect Biden Announces American Rescue Plan (Jan. 14, 2021), https://buildbackbetter.gov/wp-content/uploads/2021/01/COVID_Relief-Package-Fact-Sheet.pdf.
[43] Senator Chuck Grassley, Prepared Floor Remarks by U.S. Senator Chuck Grassley of Iowa
Celebrating Whistleblower Appreciation Day (Jul. 30, 2020), https://www.grassley.senate.gov/news/news-releases/grassley-celebrating-whistleblower-appreciation-day.
[44] United States v. Mark Schena, Indictment (Jun. 8, 2020), https://www.justice.gov/opa/press-release/file/1283931/download.
[45] U.S. Dep’t of Justice, Principal Deputy Assistant Attorney General Ethan P. Davis delivers remarks on the False Claims Act at the U.S. Chamber of Commerce’s Institute for Legal Reform (June 26, 2020), https://www.justice.gov/civil/speech/principal-deputy-assistant-attorney-general-ethan-p-davis-delivers-remarks-false-claims.
[46] State False Claims Act Reviews, Dep’t of Health & Human Servs.–Office of Inspector Gen., https://oig.hhs.gov/fraud/state-false-claims-act-reviews/index.asp.
[47] Id.
[48] Id.
[49] Id.
[50] National Whistleblower Center, The False Claims Act, https://www.whistleblowers.org/protect-the-false-claims-act/.
[51] DC B23-0035, 23d Council (2019-2020), https://lims.dccouncil.us/Legislation/B23-0035.
[52] See D.C. Code § 2-381.02(a) (2013).
[53] See D.C. Code § 2-381.02(d) (2013) (stating that “[t]his section shall not apply to claims, records, or statements made pursuant to those portions of Title 47 of the District of Columbia Official Code that refer or relate to taxation”).
[54] N.Y. State Fin. Law section § 189; 740 Ill. Comp. Stat. 175/3(c).
[55] See HB 2352 Pennsylvania General Assembly Bill Information (2019-2020), here.
[56] Id.
[57] Id.
[58] Id.
[59] Id.; Press Release, Penn. State Rep. Seth Grove, House Advances Amended Grove Bill to Protect Small Businesses and Combat Fraud (Sept. 30, 2020), http://www.repgrove.com/News/18371/Latest-News/House-Advances-Amended-Grove-Bill-to-Protect-Small-Businesses-and-Combat-Fraud.
[60] Universal Health Servs. v. United States ex rel. Escobar, 136 S. Ct. 1989, 2002 (2016).
[61] See AB-1270 False Claims Act, California Legislative Information (2019-2020), https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200AB1270.
[62] Id.
[63] See AB-2570 False Claims Act, California Legislative Information (2019-2020) http://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200AB2570.
The following Gibson Dunn lawyers assisted in the preparation of this article: John Partridge, James Zelenay, Jonathan Phillips, Ryan Bergsieker, Sean Twomey, Reid Rector, Allison Chapin, Michael Dziuban, Jasper Hicks, Julie Hamilton and Eva Michaels.
Gibson Dunn lawyers regularly counsel clients on the False Claims Act issues. Please feel free to contact the Gibson Dunn lawyer with whom you usually work, the authors, or any of the following members of the firm’s False Claims Act/Qui Tam Defense Group:
Washington, D.C.
F. Joseph Warin (+1 202-887-3609, fwarin@gibsondunn.com)
Joseph D. West (+1 202-955-8658, jwest@gibsondunn.com)
Andrew S. Tulumello (+1 202-955-8657, atulumello@gibsondunn.com)
Karen L. Manos (+1 202-955-8536, kmanos@gibsondunn.com)
Jonathan M. Phillips (+1 202-887-3546, jphillips@gibsondunn.com)
Geoffrey M. Sigler (+1 202-887-3752, gsigler@gibsondunn.com)
New York
Zainab N. Ahmad (+1 212-351-2609, zahmad@gibsondunn.com)
Matthew L. Biben (+1 212-351-6300, mbiben@gibsondunn.com)
Reed Brodsky (+1 212-351-5334, rbrodsky@gibsondunn.com)
Alexander H. Southwell (+1 212-351-3981, asouthwell@gibsondunn.com)
Denver
Robert C. Blume (+1 303-298-5758, rblume@gibsondunn.com)
Monica K. Loseman (+1 303-298-5784, mloseman@gibsondunn.com)
John D.W. Partridge (+1 303-298-5931, jpartridge@gibsondunn.com)
Ryan T. Bergsieker (+1 303-298-5774, rbergsieker@gibsondunn.com)
Dallas
Robert C. Walters (+1 214-698-3114, rwalters@gibsondunn.com)
Los Angeles
Nicola T. Hanna (+1 213-229-7269, nhanna@gibsondunn.com)
Timothy J. Hatch (+1 213-229-7368, thatch@gibsondunn.com)
James L. Zelenay Jr. (+1 213-229-7449, jzelenay@gibsondunn.com)
Deborah L. Stein (+1 213-229-7164, dstein@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650-849-5395, bwagner@gibsondunn.com)
San Francisco
Charles J. Stevens (+1 415-393-8391, cstevens@gibsondunn.com)
Winston Y. Chan (+1 415-393-8362, wchan@gibsondunn.com)
© 2021 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice.